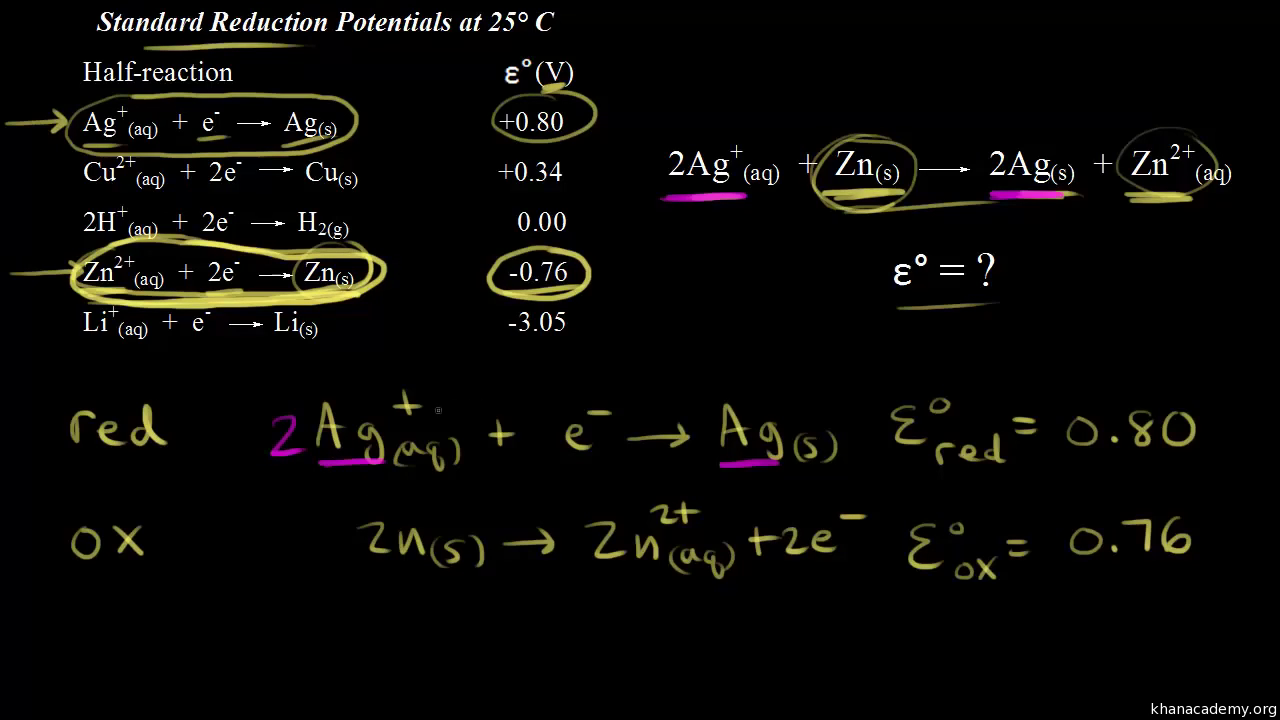
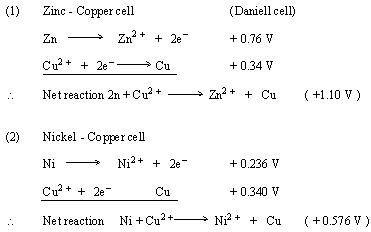
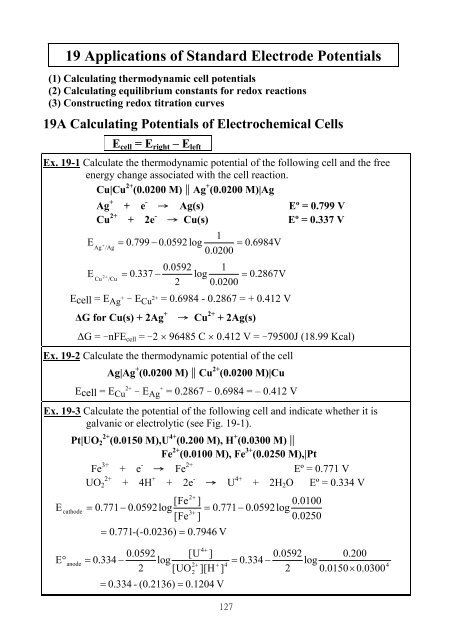
The standard electrode potential (E^∘) for Daniel cell is + 1.1 V . Calculate Δ G^∘ for the reaction. Zn(s) + Cu(aq)^2 + → Zn(aq)^2 + + Cu(s) (1 F = 96500 C/mol)

Standard Reference Electrode Standard Hydrogen Electrode (SHE) SHE: Assigned V Can be anode or cathode Pt does not take part in reaction Difficult. - ppt download

equilibrium - Calculate the cathode electrode potential in this redox reaction - Chemistry Stack Exchange

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa

Calculate the standard electrode potential of the Ni^(2+)//Ni electrode , if the cell potential ... - YouTube

Standard Cell Potential: Calculations, Electron Flow & Feasibility (5.4.2) | CIE A Level Chemistry Revision Notes 2022 | Save My Exams
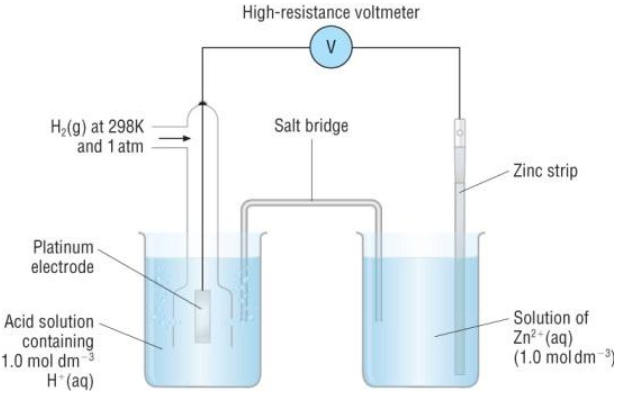
How to calculate the potential of zinc electrode capacity when in contact with 0.1M zinc sulphate solution in reference to hydrogen electrode when given the standard cell potential of Zn2 + /

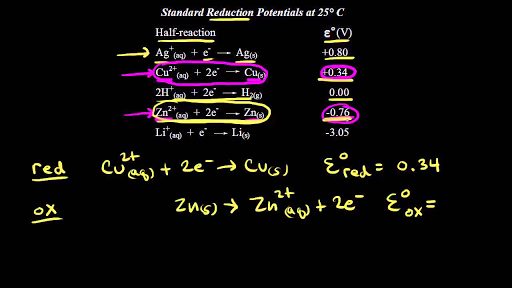
SOLVED: Calculate the standard cell potential of the following cell at 25'C: Pb(s) Pb2+ (aq) || Cu? + (aq) Cu(s) Standard Electrode (Reduction) Potentials in Aqueous Solution at 258C Cathode (Reduction) Half-Reaction
![PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar PDF] Standard Electrode Potentials and Temperature Coefficients in Water at 298.15 K | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/56964684a624c5af38c7e62256db3faa4c542d88/19-Table2-1.png)