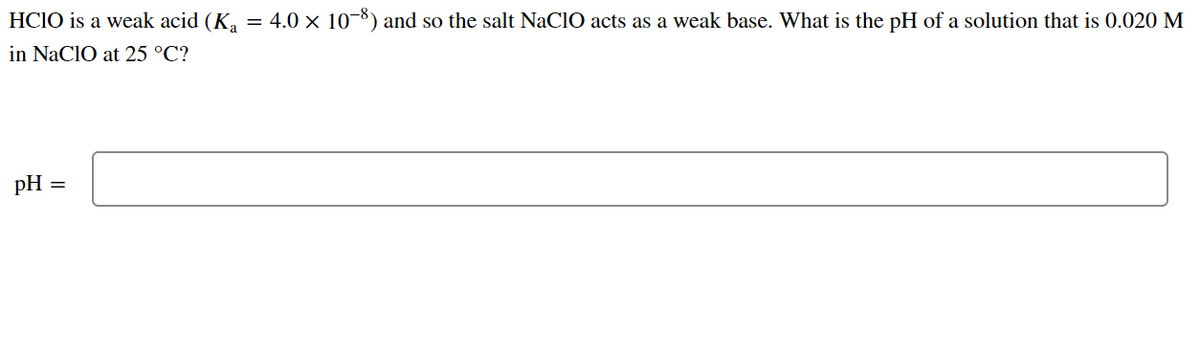
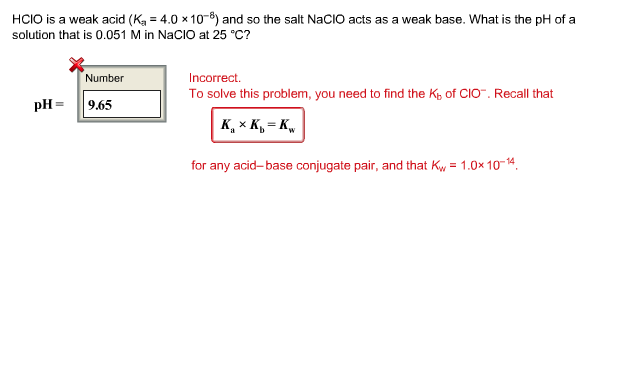
OneClass: HClO is a weak acid (Ka = 4x10^-8) and so the salt NaClO acts as a weak base. What is the p...
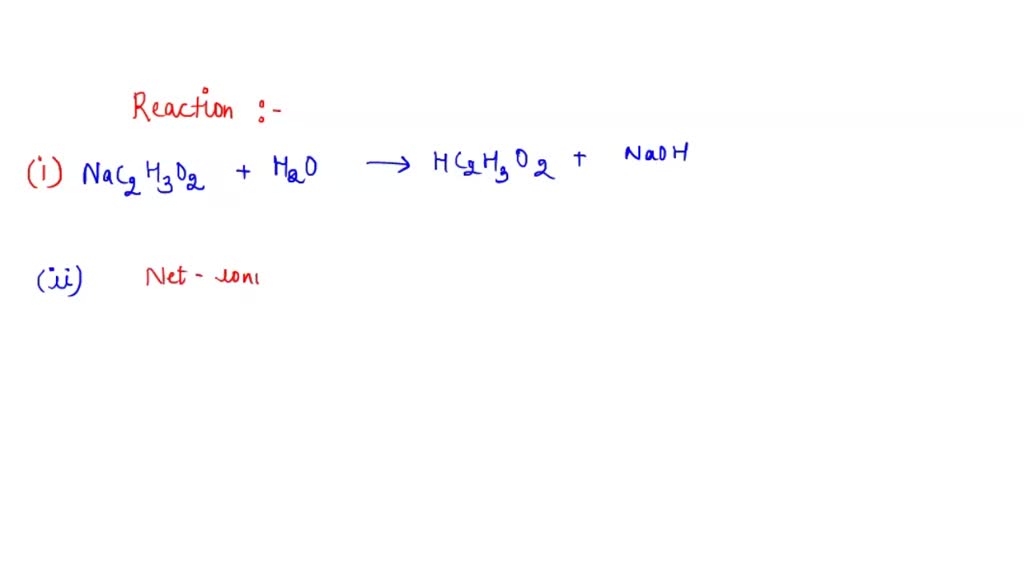
SOLVED: What is the dissolution reaction for solid NaClO?Once the ionic solid has dissolved, the anion that is formed is able to react as a base, with water as the acid. What

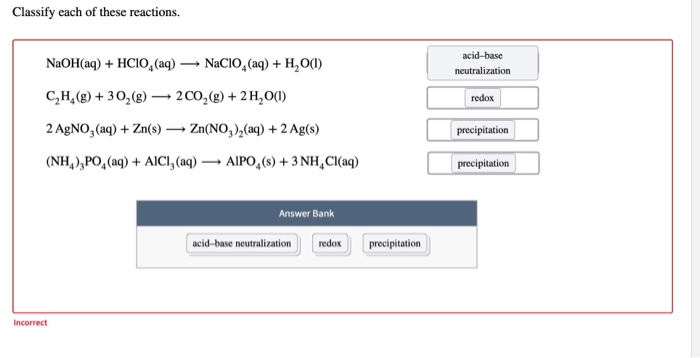
Acids, Bases, and Salts You should be able to Understand the acid-base theories of Arrhenius, Brønsted-Lowry, and Lewis. Identify strong acids and. - ppt download

HClO is a weak acid (Ka = 4.0 x 10-8) and so the salt NaClO acts as a weak base. What is the pH of a solution that is 0.034 M in

Lab 24 - Hydrolysis A salt formed between a strong acid and a weak base is an acid salt. Ammonia is a weak base, and its salt with any strong acid gives. -
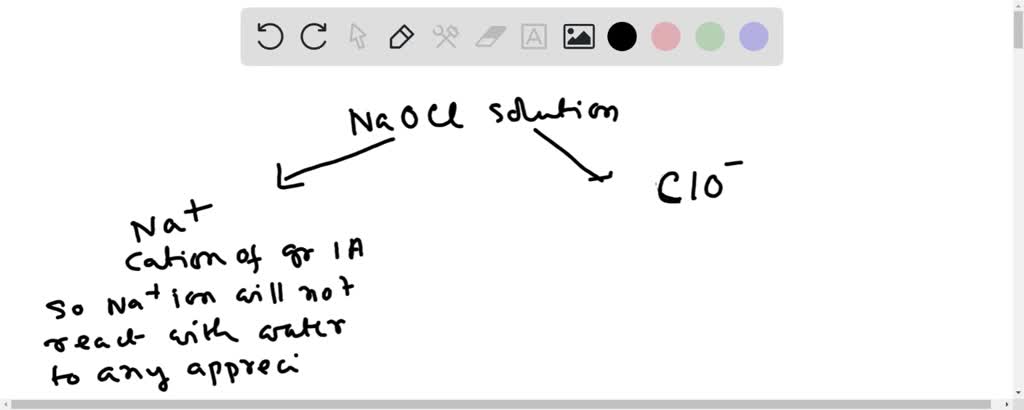
SOLVED: a) Why do salts from a strong base and a weak acid give a basic solution in aqueous solution? Use sodium hypochlorite (NaClO), used as a disinfectant, to illustrate this b)Why
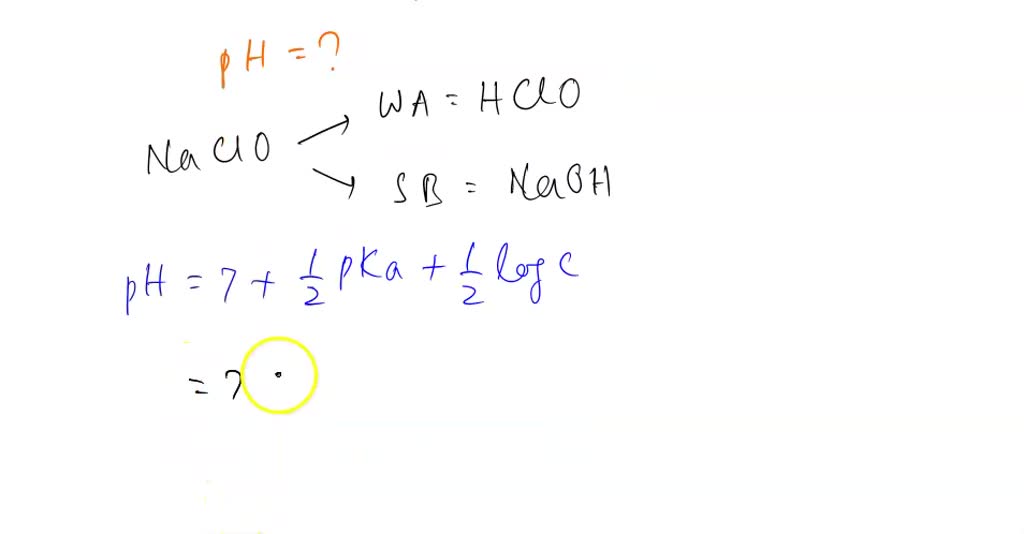








![Is NaOCl Acidic or Basic [Acids and Bases] - YouTube Is NaOCl Acidic or Basic [Acids and Bases] - YouTube](https://i.ytimg.com/vi/HXJWALr3BEY/maxresdefault.jpg)

![47 Chemistry QPack] How do you know that HClO and NaClO are conjugates of each other? : r/Mcat 47 Chemistry QPack] How do you know that HClO and NaClO are conjugates of each other? : r/Mcat](https://preview.redd.it/8rsg2yxkum831.jpg?width=640&crop=smart&auto=webp&s=ab0ed8cf6296dab3cf389a686d1f90e42e9178b8)