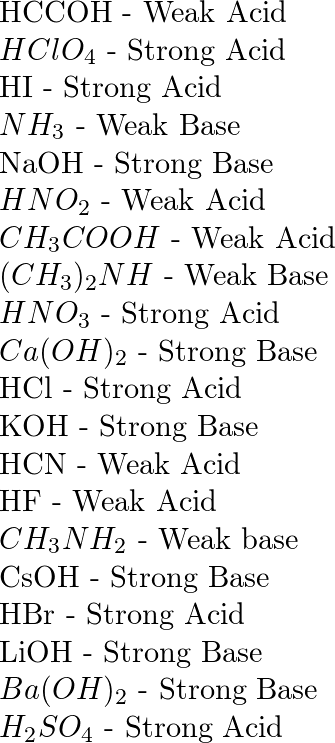Titration curves for solutions with 1.0 × 10 −4 moles of LiOH·H 2 O and... | Download Scientific Diagram

How to Identify the Major Species in a Mixture of Weak and Strong Acids or Bases | Chemistry | Study.com
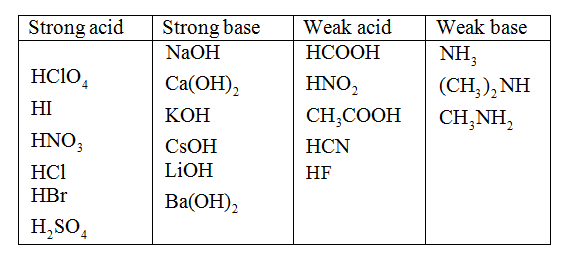
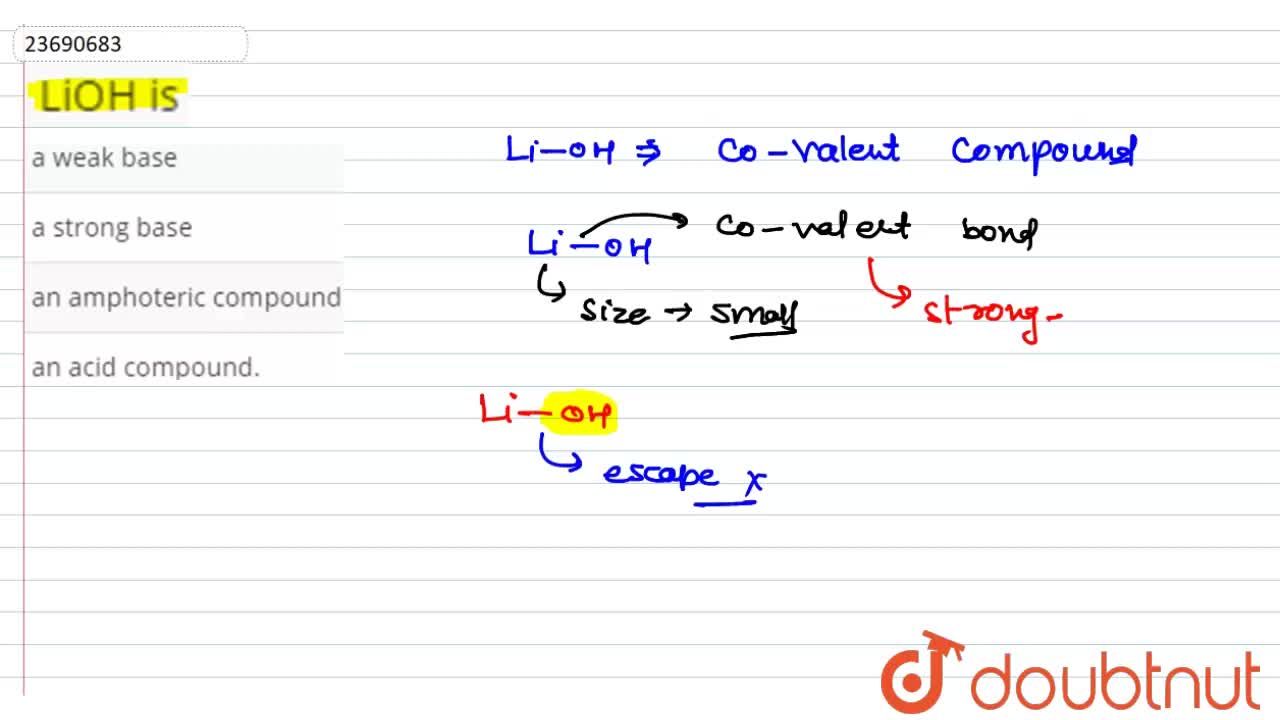
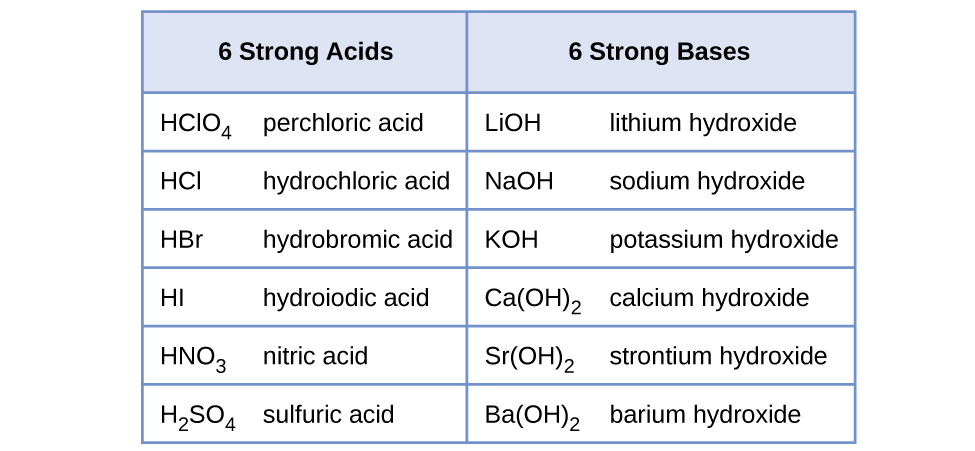
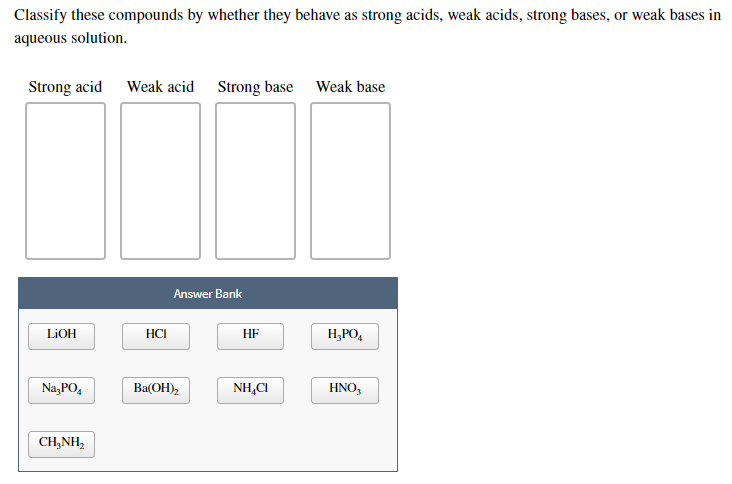
Classify each substance as a strong acid, strong base, weak acid, or weak base - Home Work Help - Learn CBSE Forum

SOLVED:Name each of the following acids or bases: a. H3 PO4 b. LiOH c. HI d. HCN e. HCIO2 f. Ca(OH)2

Equilibrium – Acids and Bases. Review of Acids and Bases Arrhenius Theory of Acids and Bases ▫An acid is a substance that dissociates in water to produce. - ppt download
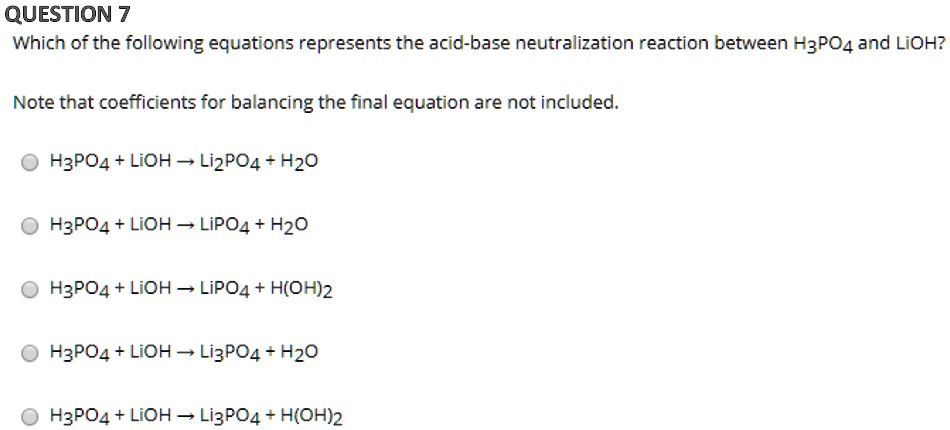
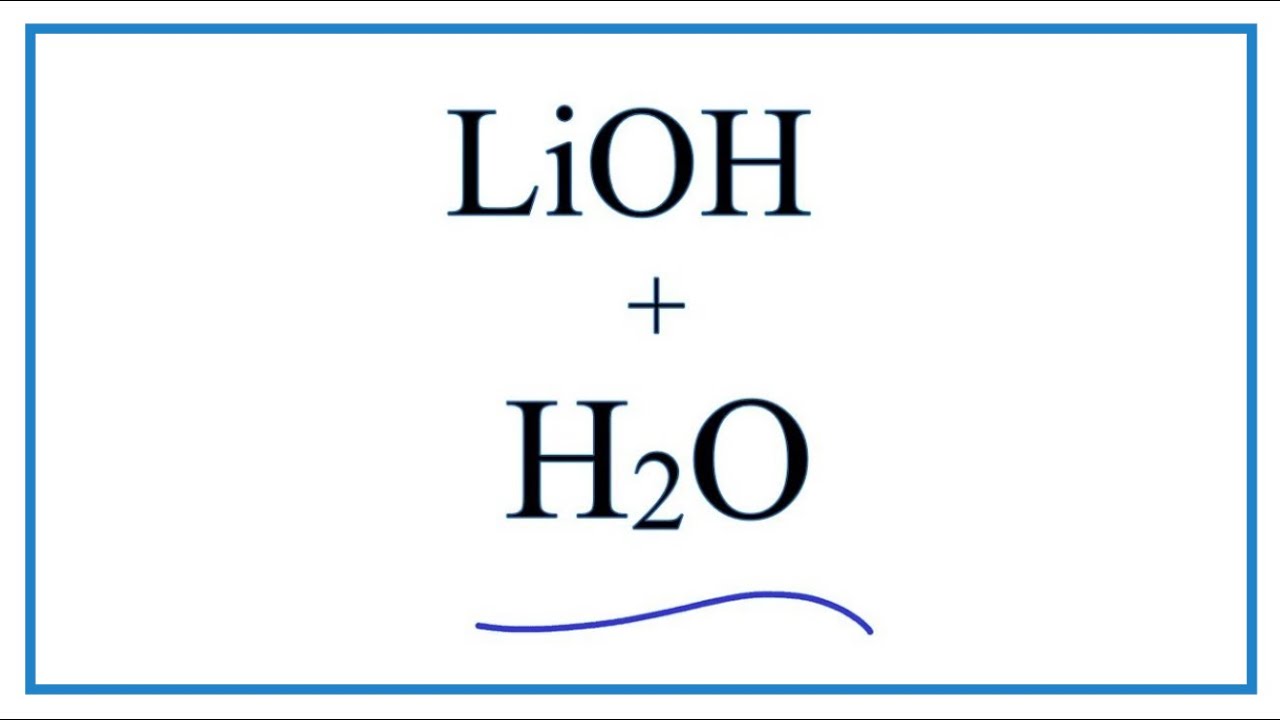
SOLVED: QUESTION 7 Which of the following equations represents the acid-base neutralization reaction between H3PO4 ad LiOH? Note that coefficients for balancing the final equation are not included: H3PO4 + LiOH LizPO4 -