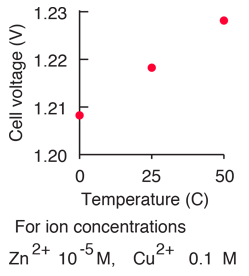
Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in 2.0 MFe^2 and 0.02 M FE^3 solution. Given E^0Fe^3/Fe^+2 = 0.771 V .

Question Video: Calculating a Cell Potential from Standard Electrode Potentials of Cadmium and Nickel | Nagwa

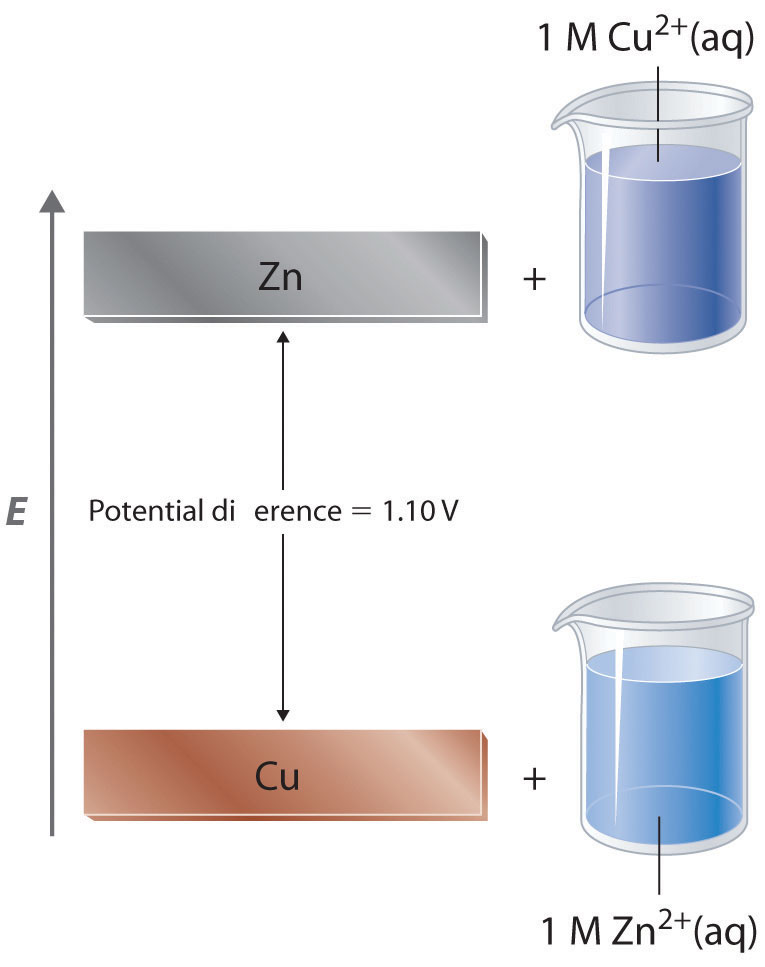
equilibrium - Calculate the cathode electrode potential in this redox reaction - Chemistry Stack Exchange

OneClass: Given the following half-reactions and their respective standard reduction potentials calcu...
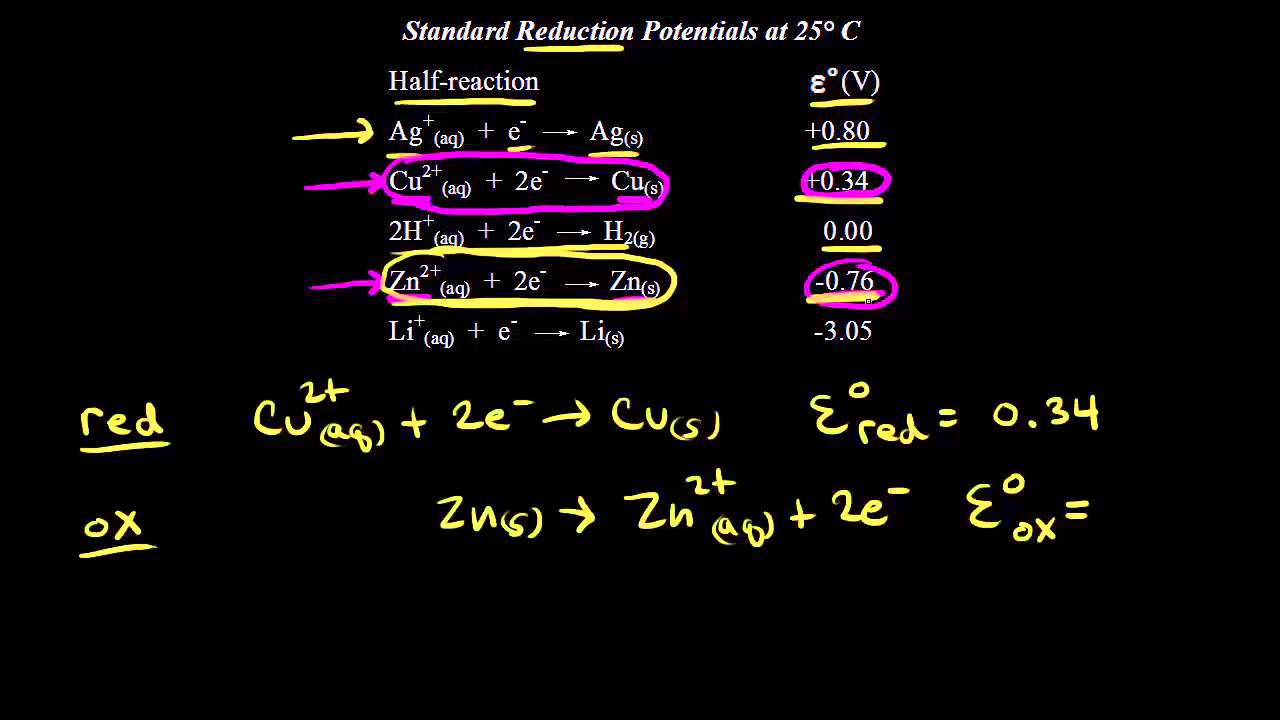
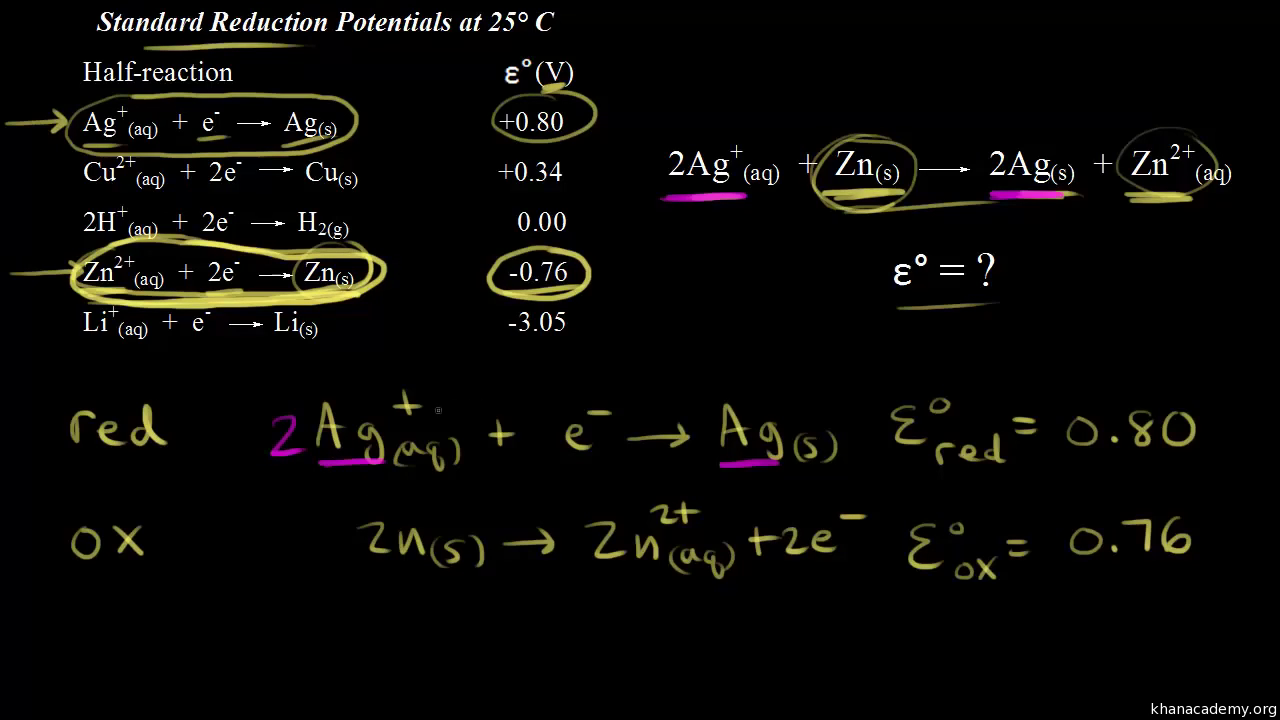
Standard reduction potentials | Redox reactions and electrochemistry | Chemistry | Khan Academy - YouTube
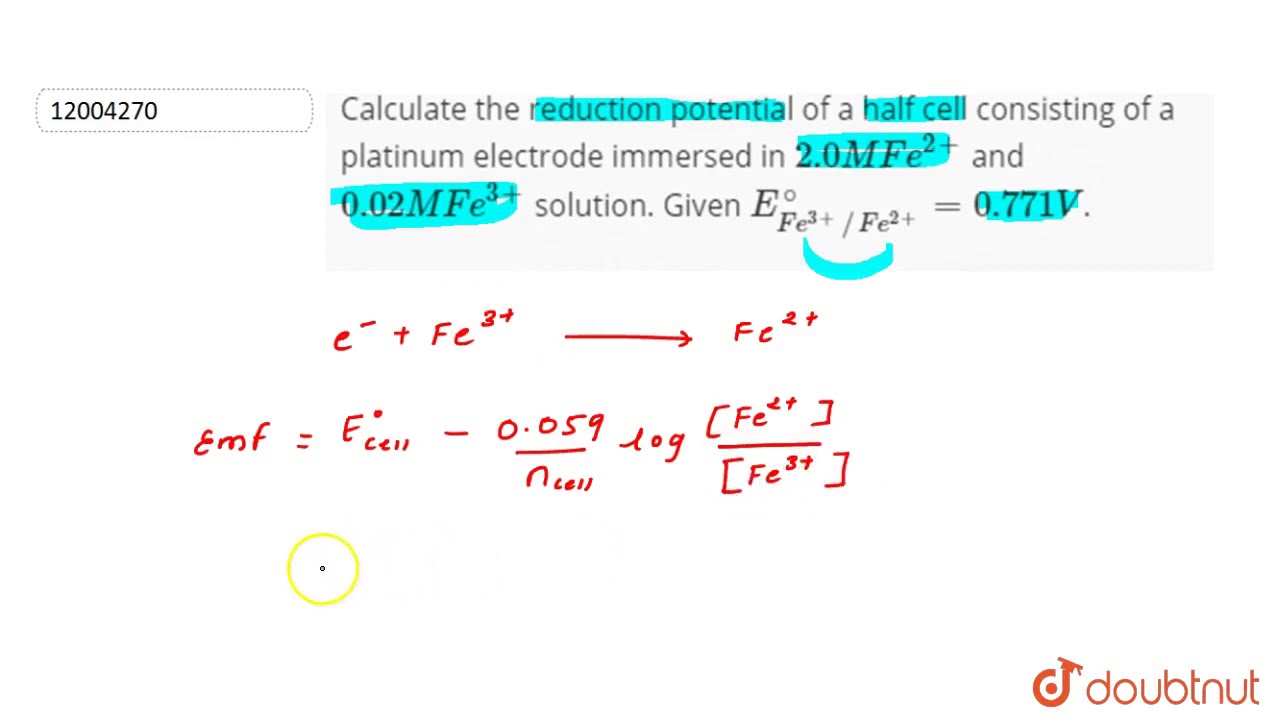
Calculate the reduction potential of a half cell consisting of a platinum electrode immersed in - YouTube
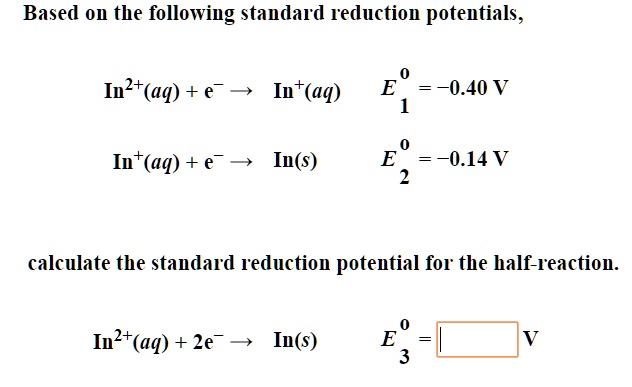
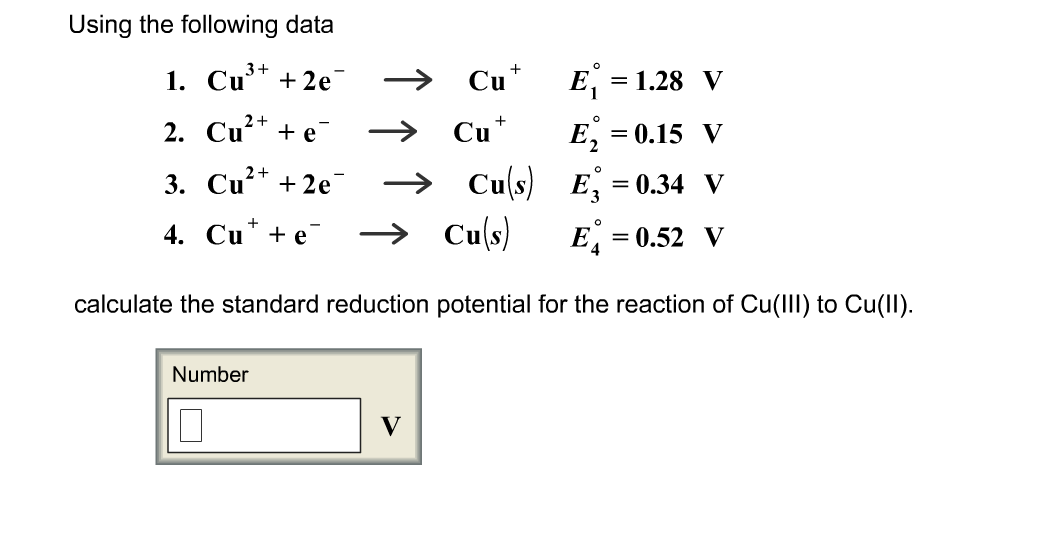
SOLVED: Based on the following standard reduction potentials, Iu2t(aq) + e" Int(aq) =-0.40 V Int(aq) + e- 5 In(s) E =-0.14V calculate the standard reduction potential for the half-reaction Iult(aq) + 2e"

Question Video: Calculating the Standard Cell Potential for a Magnesium/Silver Galvanic Cell | Nagwa