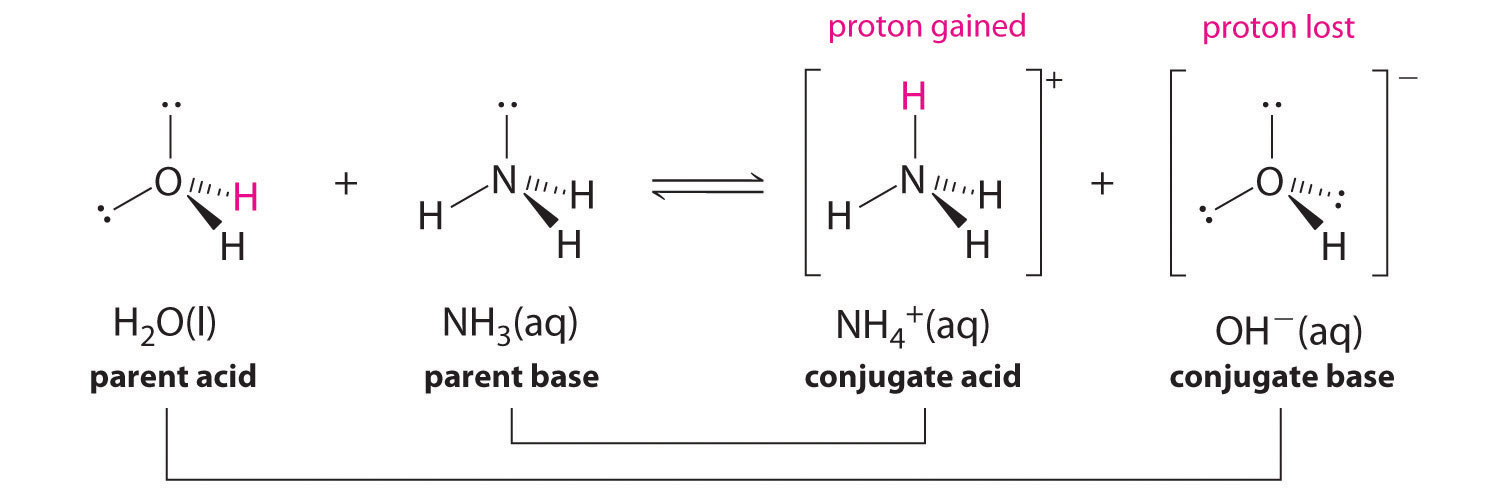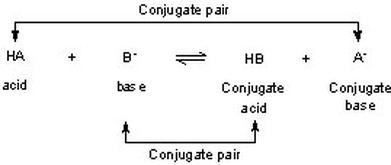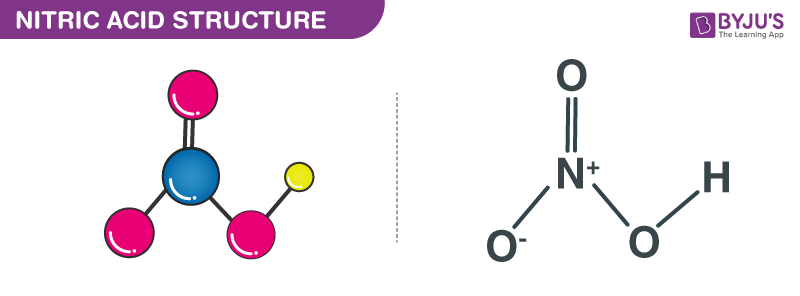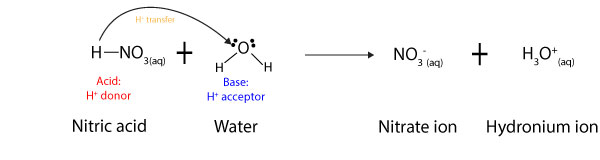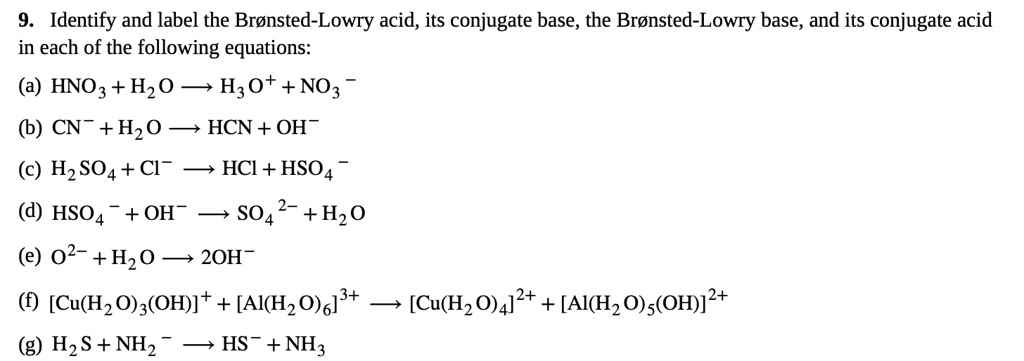
SOLVED: Identify and label the Bronsted-Lowry acid, its conjugate base, the Bronsted-Lowry base, and its conjugate acid in each of the following equations: (a) HNO3 + Hz0 HzO++ NO3 (6) CN: +Hz0
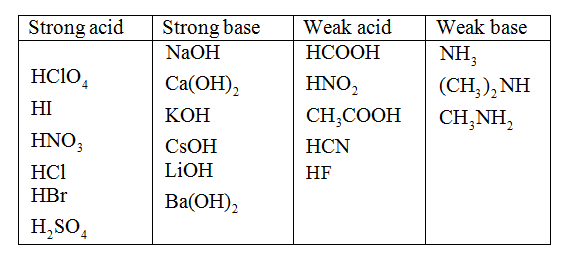
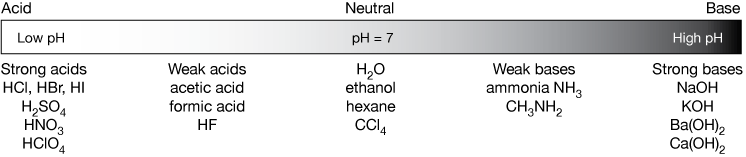
Classify each substance as a strong acid, strong base, weak acid, or weak base - Home Work Help - Learn CBSE Forum