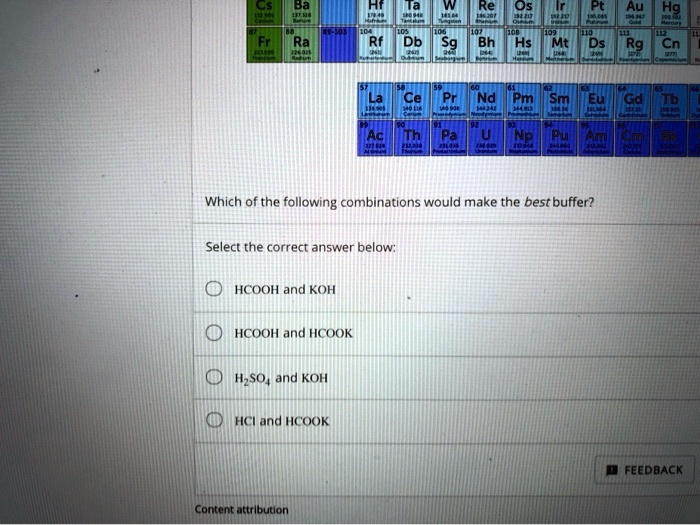
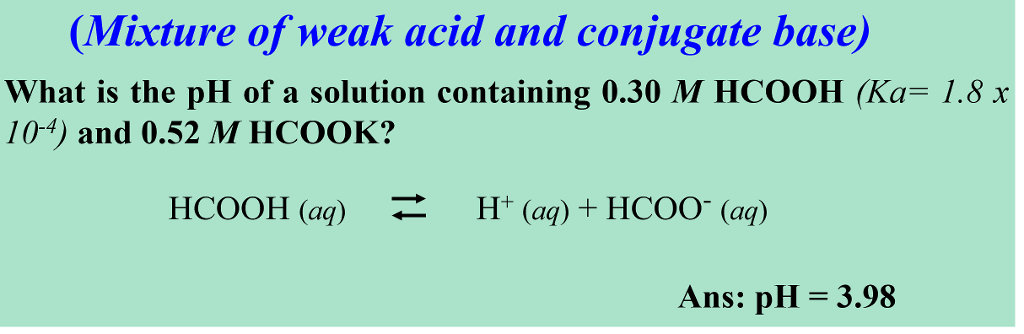
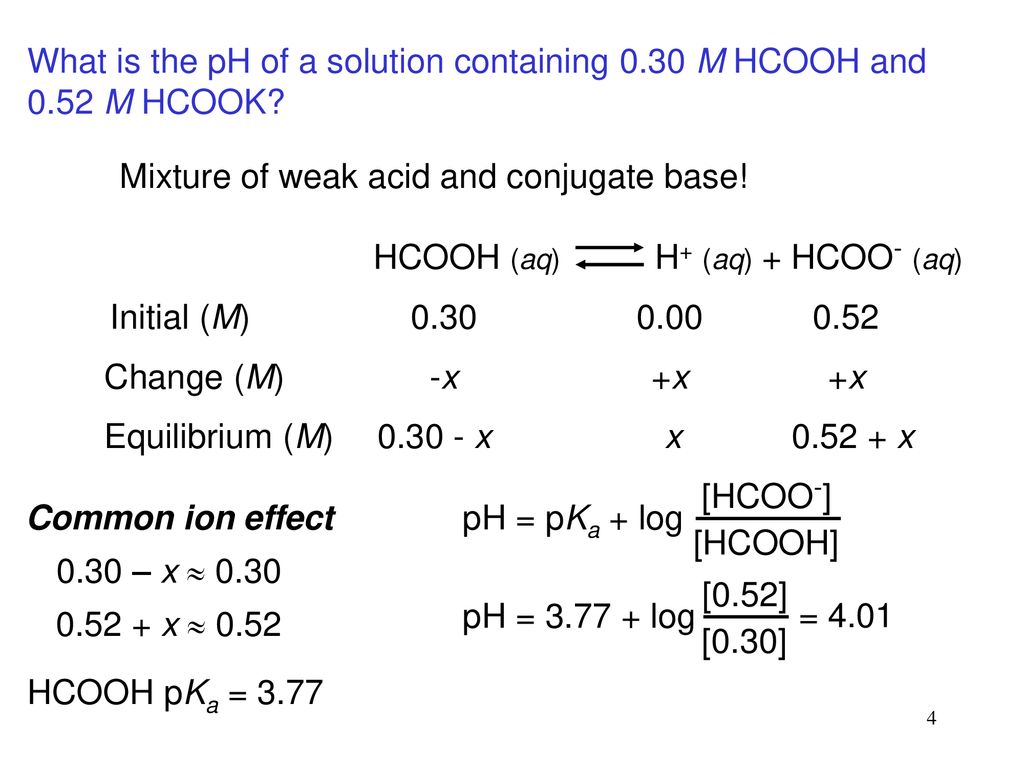
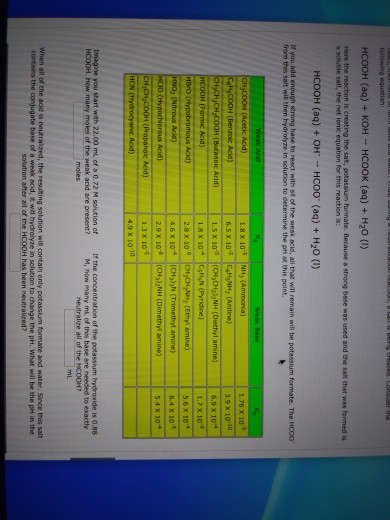
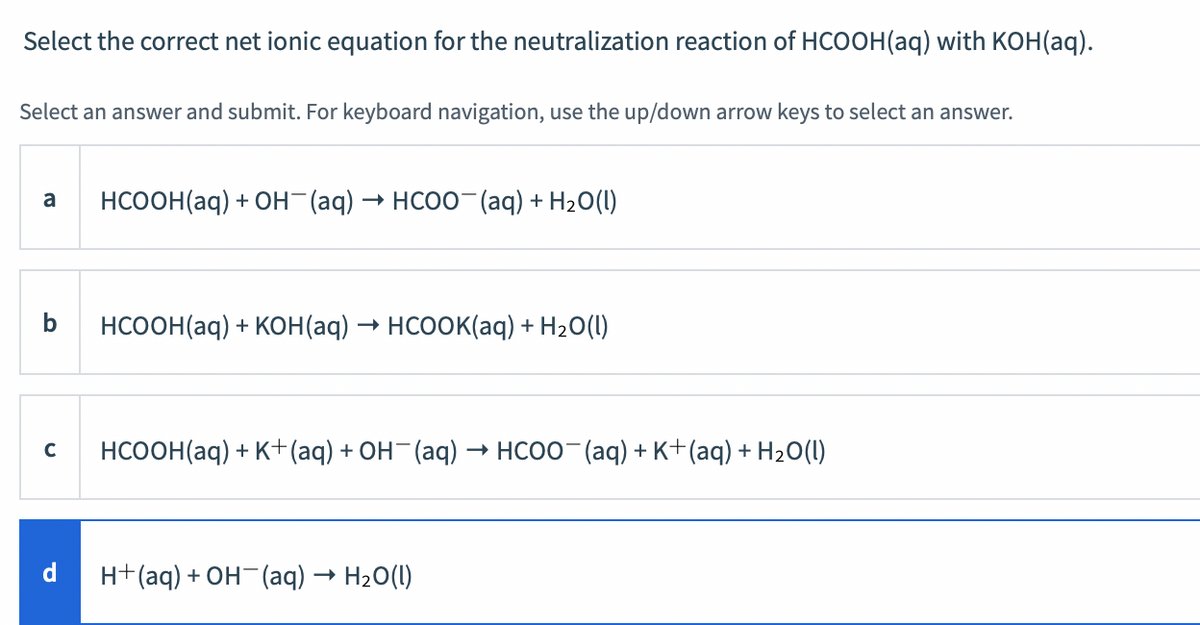
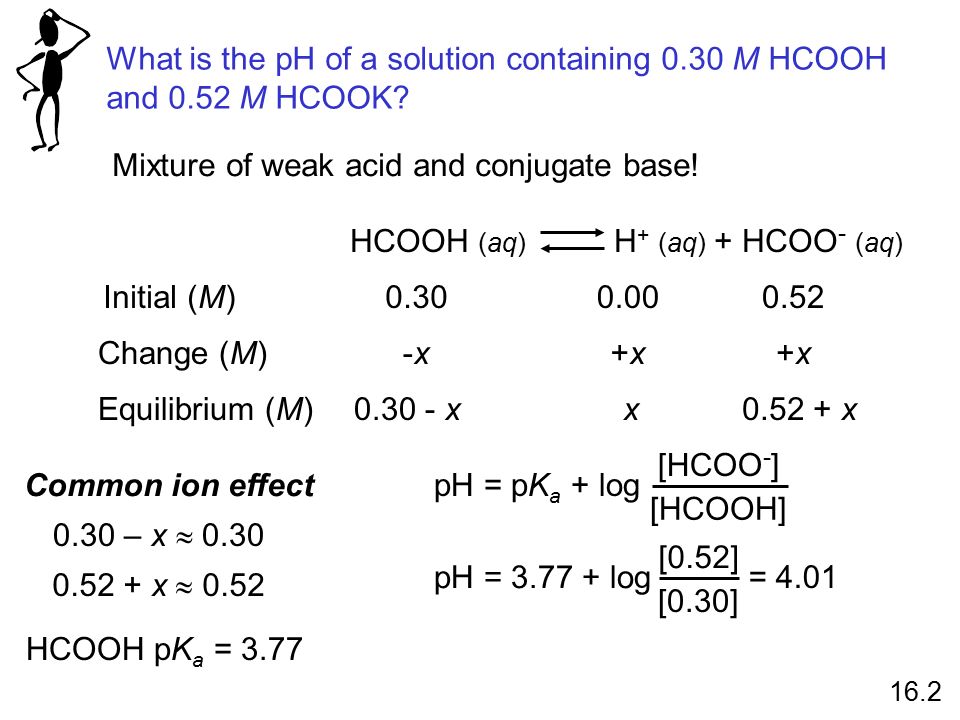
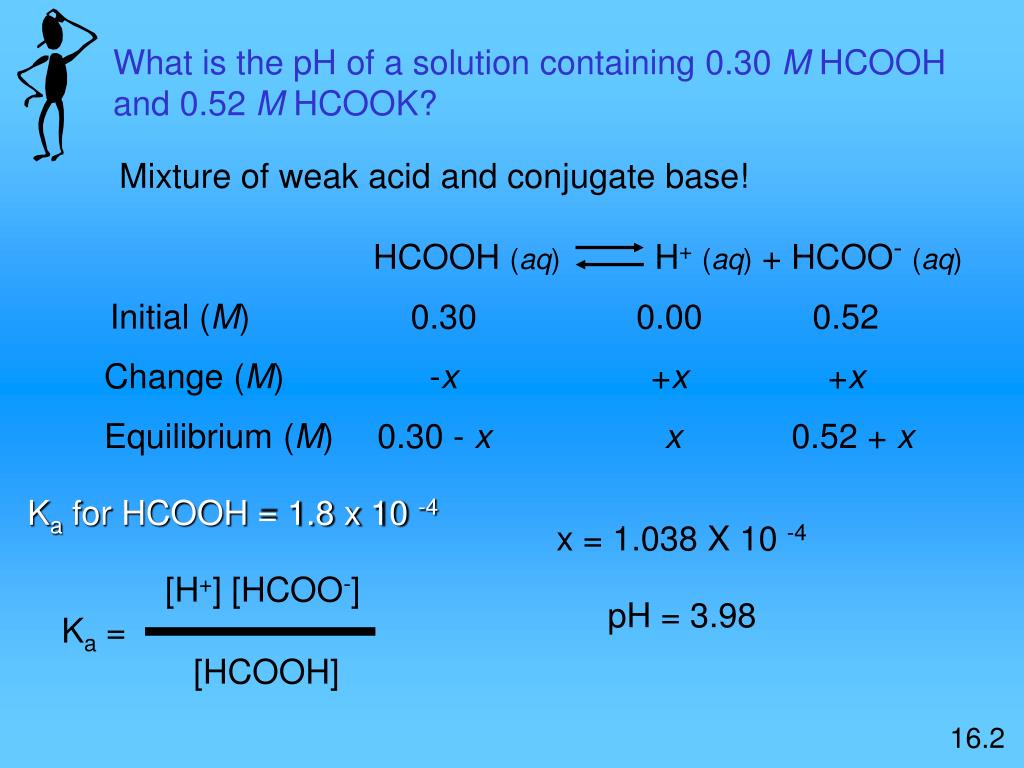
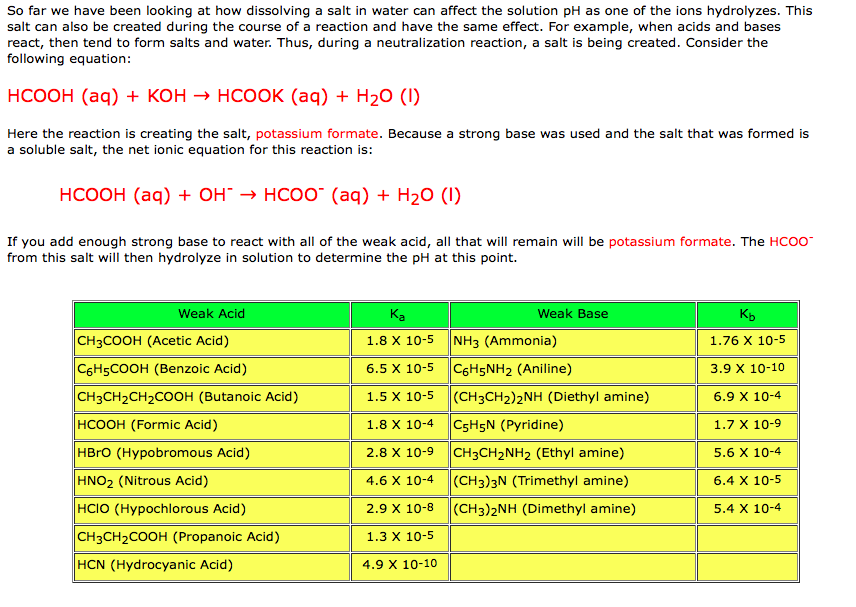
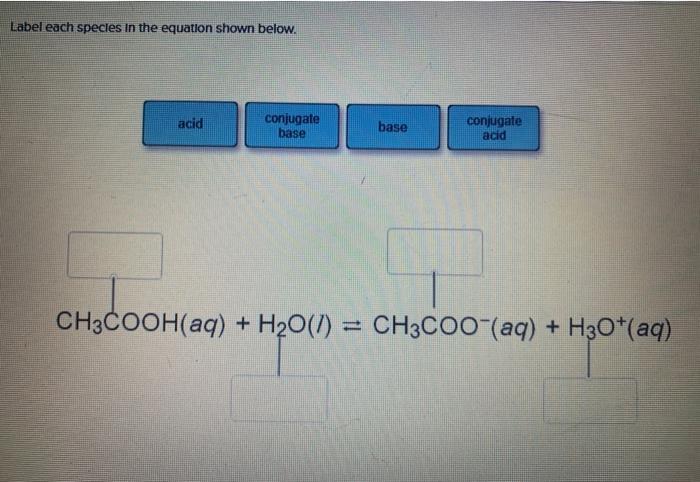
In acid buffer solution (pH = 4.4), the ratio of concentrations of acid to salt is 2 : 1. The value of dissociation constant of weak acid may be:
A 0.05M HCOOH and 0.1M HCOOK buffer solution was diluted 100 times. How did pH change and why? - Quora

SOLVED: State whether aqueous solutions of the following salts produce acidic, basic or neutral solutions Justify your choice by referring to the specific properties of BOTH the cation and anion. You must

Why an aqueous solution of NH4Cl is acidic while that of HCOOK is basic - Chemistry - Ionic Equilibria - 16488273 | Meritnation.com

EHSQ (Environment,Health,Safety and Quality) : Question : Is HCOOK an acid or base or neutral ? Answer : HCOOK ( Potassium formate ) is base
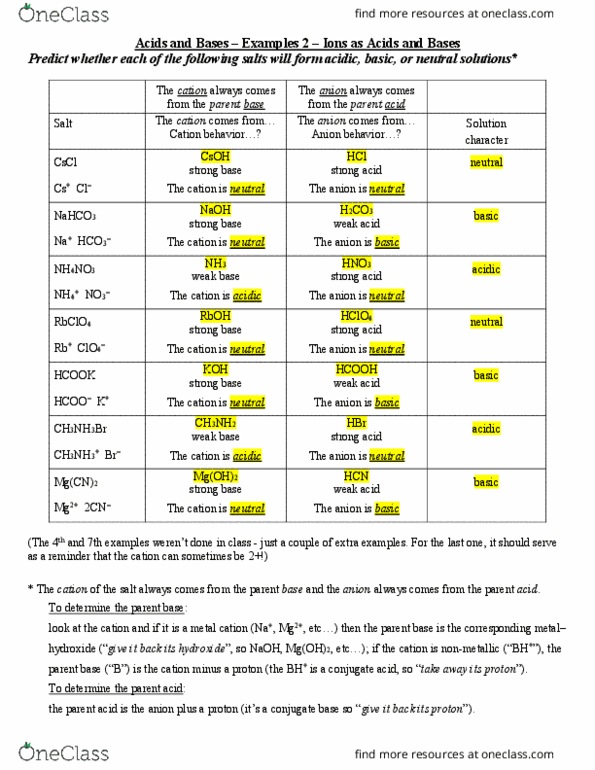
CHE 1302 Lecture Notes - Fall 2017, Lecture 12 - Equilibrium Constant, Hydrofluoric Acid, Rice Chart

Effect of HCOOK/Ethanol on Fe/HUSY, Ni/HUSY, and Ni–Fe/HUSY Catalysts on Lignin Depolymerization to Benzyl Alcohols and Bioaromatics | ACS Omega
![pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download](https://images.slideplayer.com/26/8472866/slides/slide_40.jpg)
pH = - log [H + ] or pH = - log [H 3 O + ] Example: If [H + ] = 1 X pH = - log 1 X pH = - (- 10) pH = 10 What would be the pH of a. - ppt download

Acid-Base Equilibria Chapter HF( aq ) + H 2 O( l ) H 3 O + ( aq ) + F - ( aq ) Addition of NaF will shift the equilibrium to the _______because. - ppt download
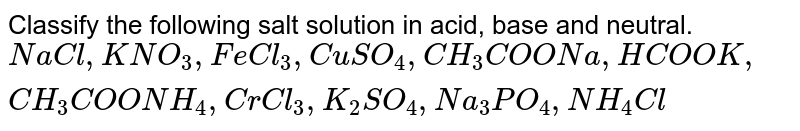
Classify the following salt solution in acid, base and neutral. NaCl, KNO3 , FeCl3 , CuSO4 , CH3COONa, HCOOK, CH3COONH4 , CrCl3 , K2SO4 , Na3PO4 , NH4Cl

Classify the following salt solution in acid, base and neutral. NaCl, KNO3 , FeCl3 , CuSO4 , CH3COONa, HCOOK, CH3COONH4 , CrCl3 , K2SO4 , Na3PO4 , NH4Cl

EHSQ (Environment,Health,Safety and Quality) : Question : Is HCOOK an acid or base or neutral ? Answer : HCOOK ( Potassium formate ) is base