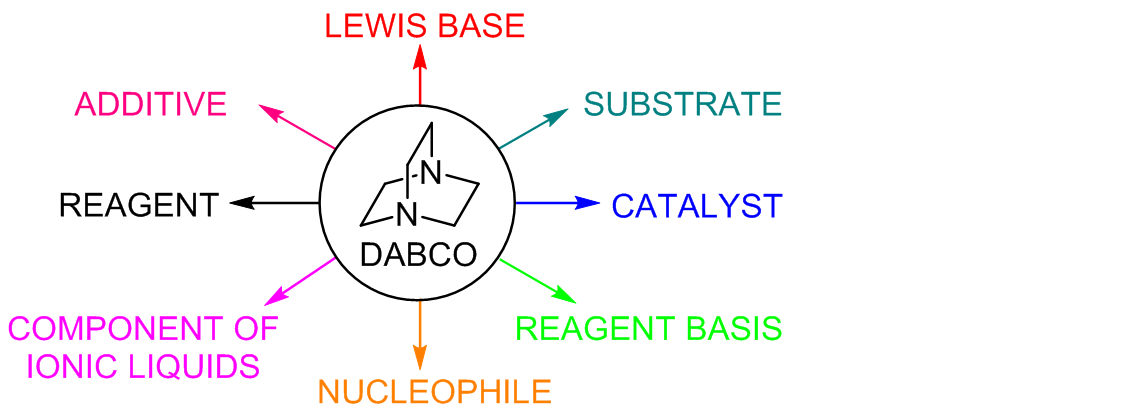
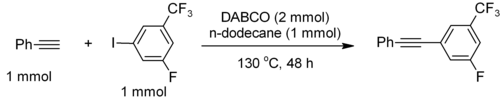DABCO‐Catalysed Amidation under Assistance of Aerial Oxidation: Access to α‐ketoamides - Monga - 2018 - ChemistrySelect - Wiley Online Library

Dual Nucleophilic Catalysis with DABCO for the N-Methylation of Indoles | The Journal of Organic Chemistry
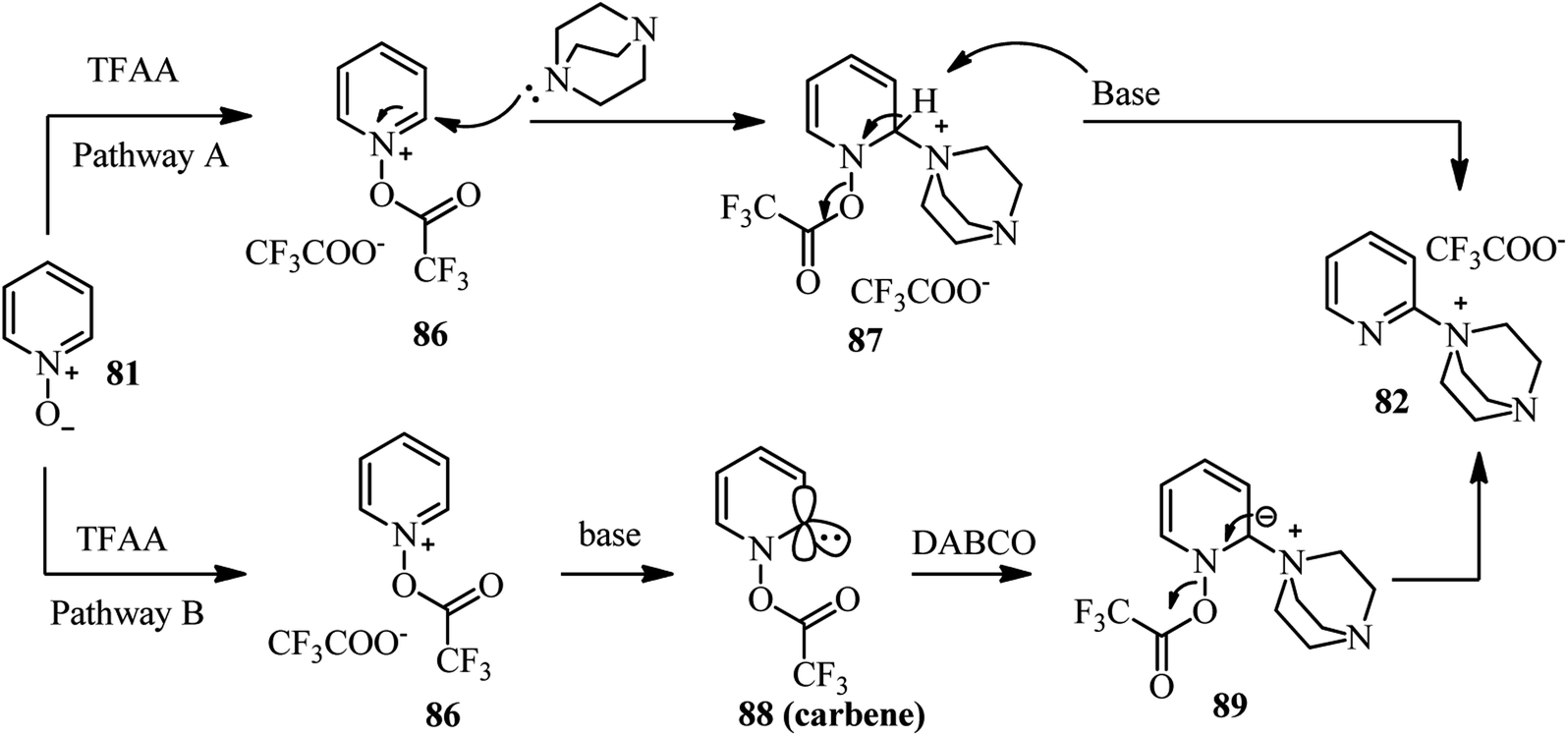
DABCO bond cleavage for the synthesis of piperazine derivatives - RSC Advances (RSC Publishing) DOI:10.1039/C9RA07870C

Nucleophilic Organic Base DABCO-Mediated Chemospecific Meinwald Rearrangement of Terminal Epoxides into Methyl Ketones | The Journal of Organic Chemistry
![1,4-Diazabicyclo[2.2.2]octane (DABCO) as a useful catalyst in organic synthesis | Bita | European Journal of Chemistry 1,4-Diazabicyclo[2.2.2]octane (DABCO) as a useful catalyst in organic synthesis | Bita | European Journal of Chemistry](http://www.eurjchem.com/public/site/images/arslanh/1_1_54_60_800.png)
1,4-Diazabicyclo[2.2.2]octane (DABCO) as a useful catalyst in organic synthesis | Bita | European Journal of Chemistry

Nucleophilic Organic Base DABCO-Mediated Chemospecific Meinwald Rearrangement of Terminal Epoxides into Methyl Ketones | The Journal of Organic Chemistry

The versatility of DABCO: synthetic applications of its basic, nucleophilic, and catalytic properties Part 1. Catalysis of Morita–Baylis–Hillman and Knoevenagel reactions | SpringerLink

Theoretical study on DABCO-catalyzed ring expansion of cyclopropyl ketone: Mechanism, chemoselectivity, and role of catalyst - ScienceDirect

Nucleophilic Organic Base DABCO-Mediated Chemospecific Meinwald Rearrangement of Terminal Epoxides into Methyl Ketones | The Journal of Organic Chemistry
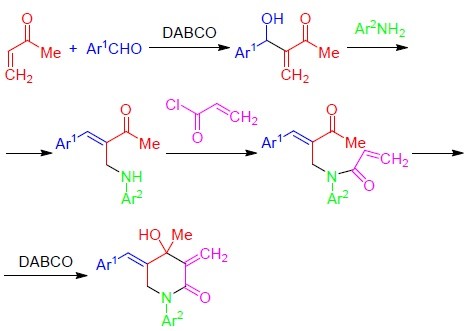
The versatility of DABCO: synthetic applications of its basic, nucleophilic, and catalytic properties Part 1. Catalysis of Morita–Baylis–Hillman and Knoevenagel reactions | SpringerLink

The versatility of DABCO: synthetic applications of its basic, nucleophilic, and catalytic properties Part 2*. Catalysis of Michael and Biginelli reactions and nucleophilic addition at C=X and C≡X bonds | SpringerLink
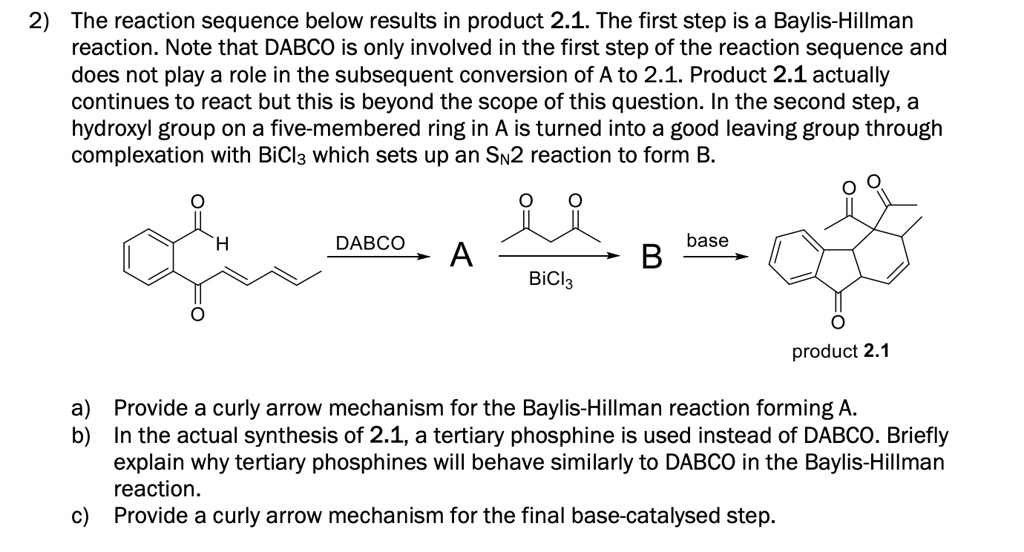
SOLVED: 2) The reaction sequence below results in product 2.1 The first step is a Baylis-Hillman reaction. Note that DABCO is only involved in the first step of the reaction sequence and

New dicationic DABCO-based ionic liquids: a scalable metal-free one-pot synthesis of bis-2-amino-5-arylidenethiazol-4-ones | Royal Society Open Science