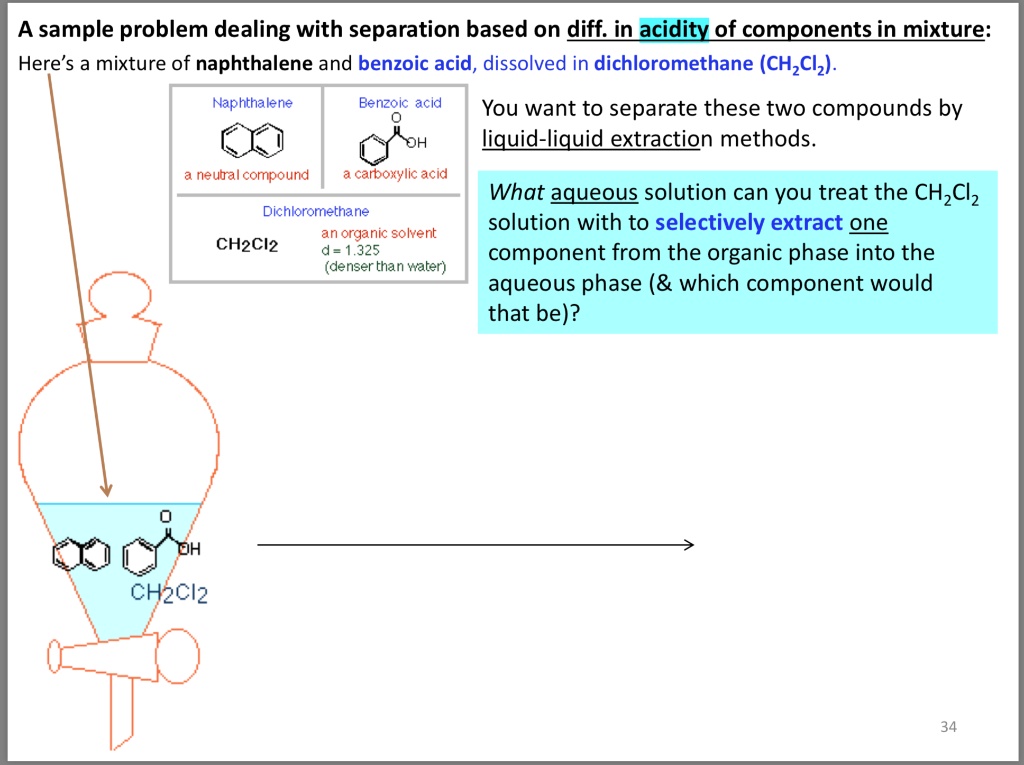
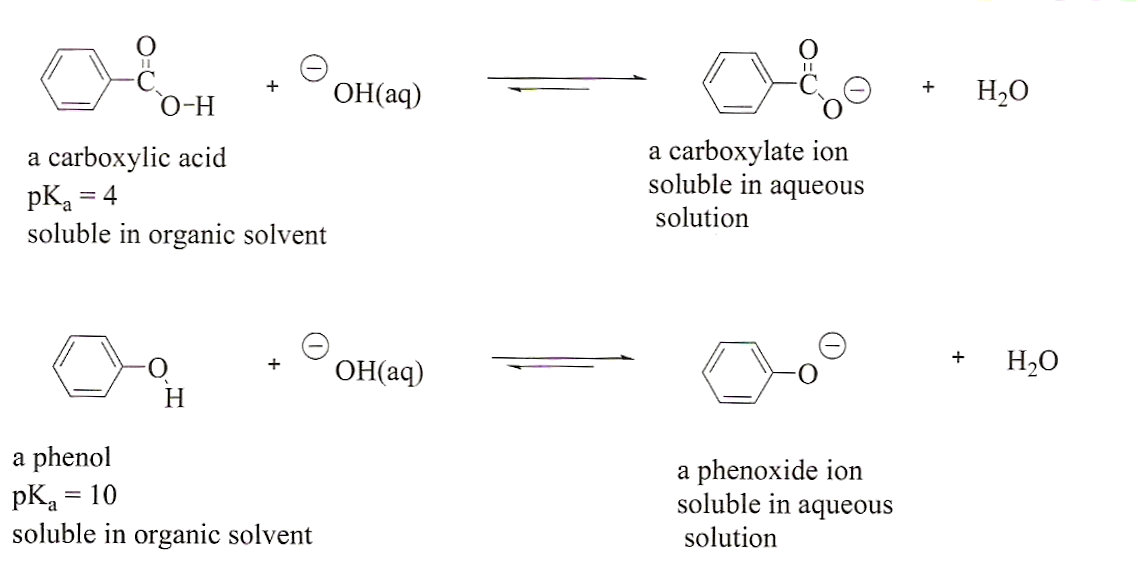
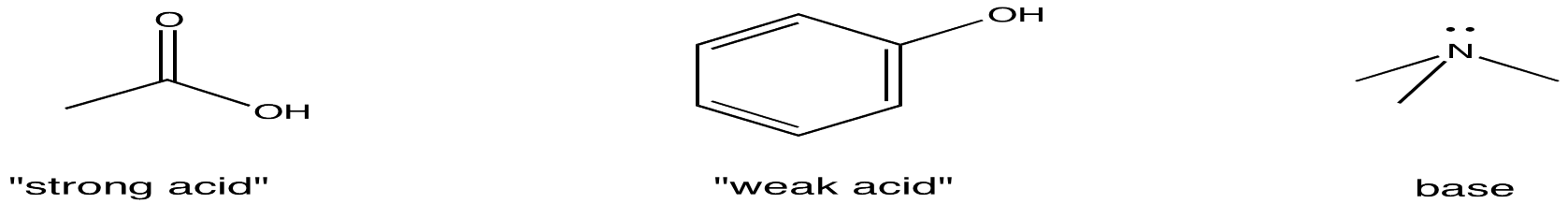
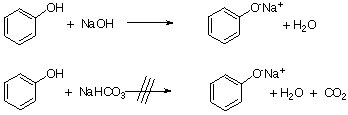
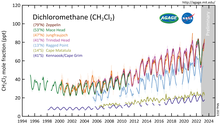
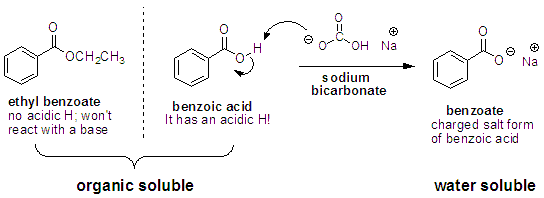
Acid-base properties of tetrapyrazinoporphyrazines. 1. Deprotonation of octaethyltetrapyrazinoporphyrazine in CH2Cl2, THF, DMSO and pyridine. The crucial role of water - ScienceDirect

Radical Reactions Induced by Visible Light in Dichloromethane Solutions of Hünig's Base: Synthetic Applications and Mechanistic Observations - Böhm - 2016 - Chemistry – A European Journal - Wiley Online Library

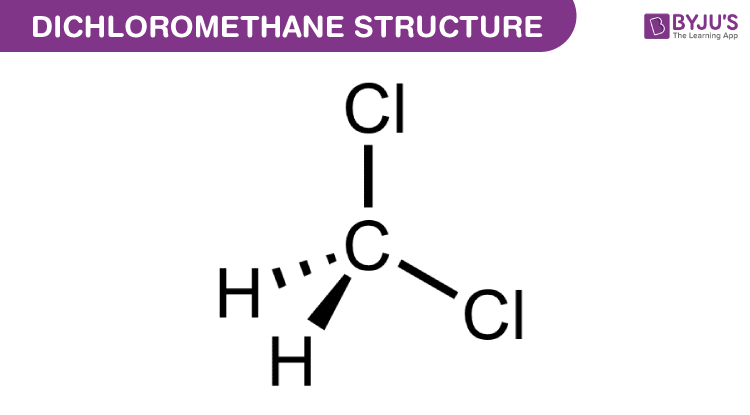
Dichloromethane as a methylene synthon for regioselective linkage of diverse carboxylic acids: Direct access to methylene diesters under metal-free conditions - ScienceDirect
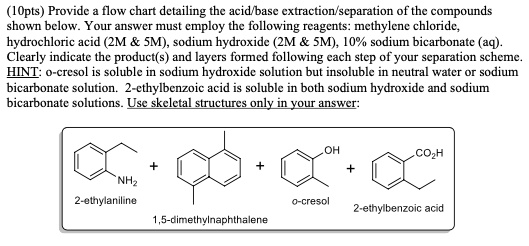
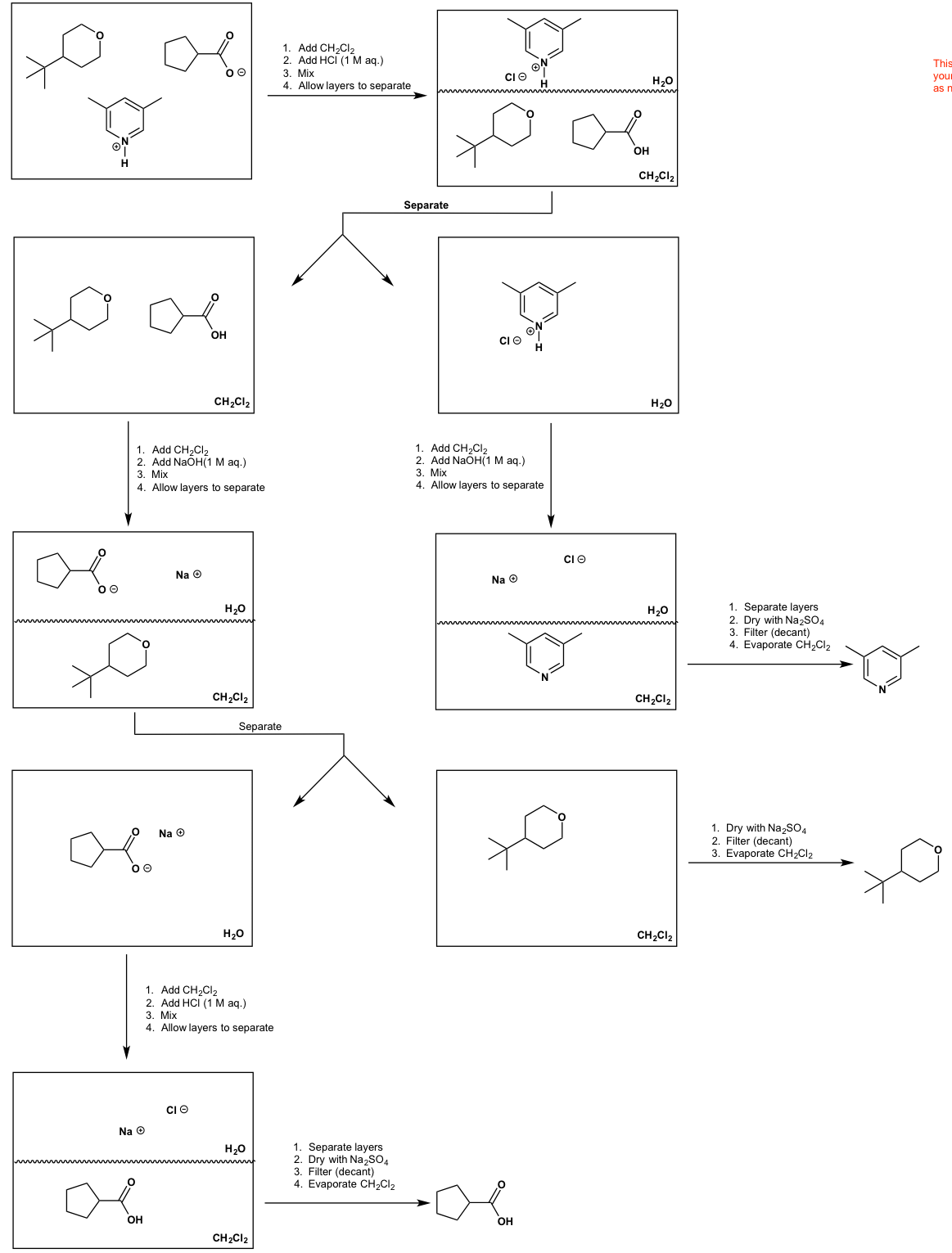
SOLVED: (1Opts) Provide flow chart detailing the acid base extraction scparation Of the compounds shown below. Your answer must employ the following reagents: methylene chloride; hydrochloric acid (ZM SM), sodium hydroxide (2M

Reaction of N,N-Dimethyltryptamine with Dichloromethane Under Common Experimental Conditions | ACS Omega
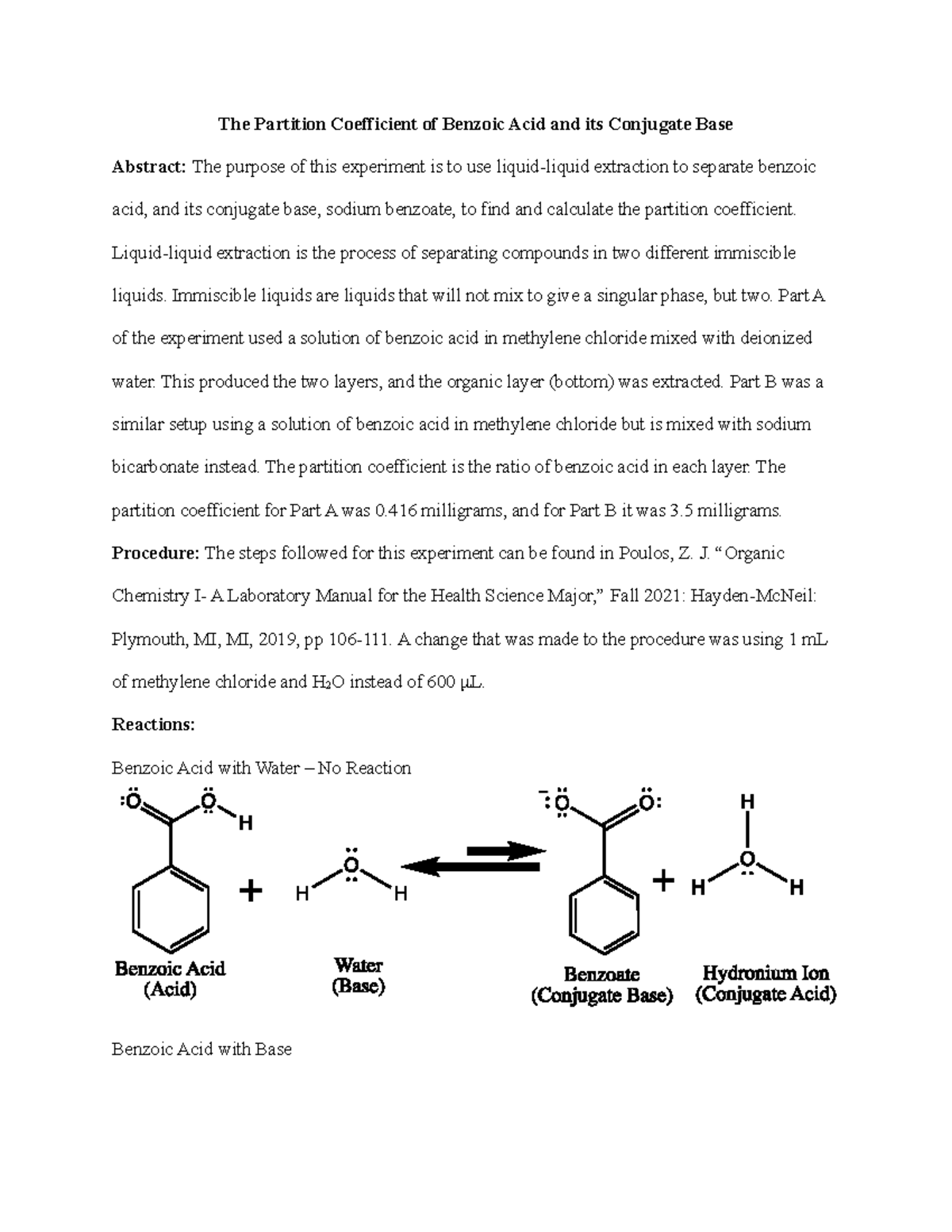
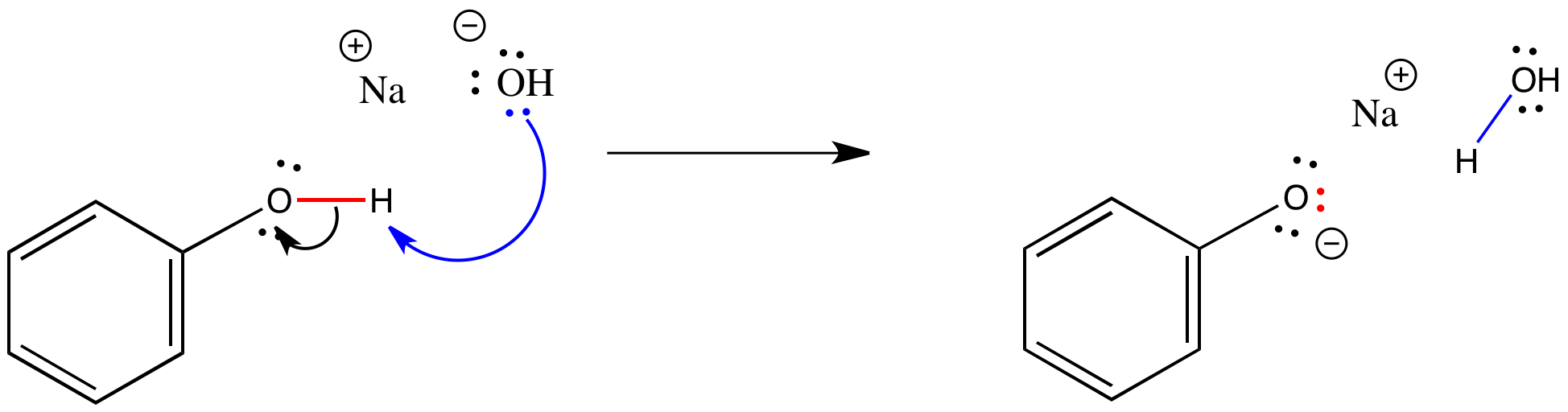
The Partition Coefficient Extraction of Benzoic Acid and its Conjugate Base Lab Report - The - Studocu

A) Acid-and base-induced structural conversion between ins-SP-PPE and... | Download Scientific Diagram
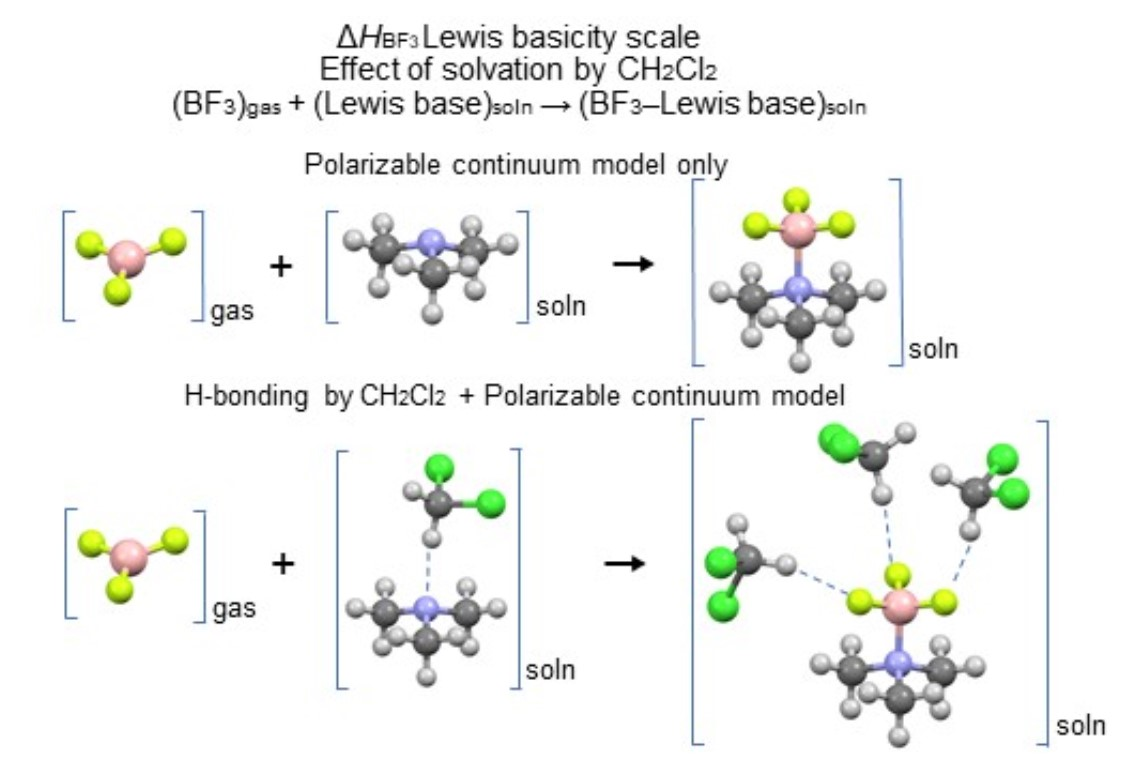
Molecules | Free Full-Text | Enthalpies of Adduct Formation between Boron Trifluoride and Selected Organic Bases in Solution: Toward an Accurate Theoretical Entry to Lewis Basicity