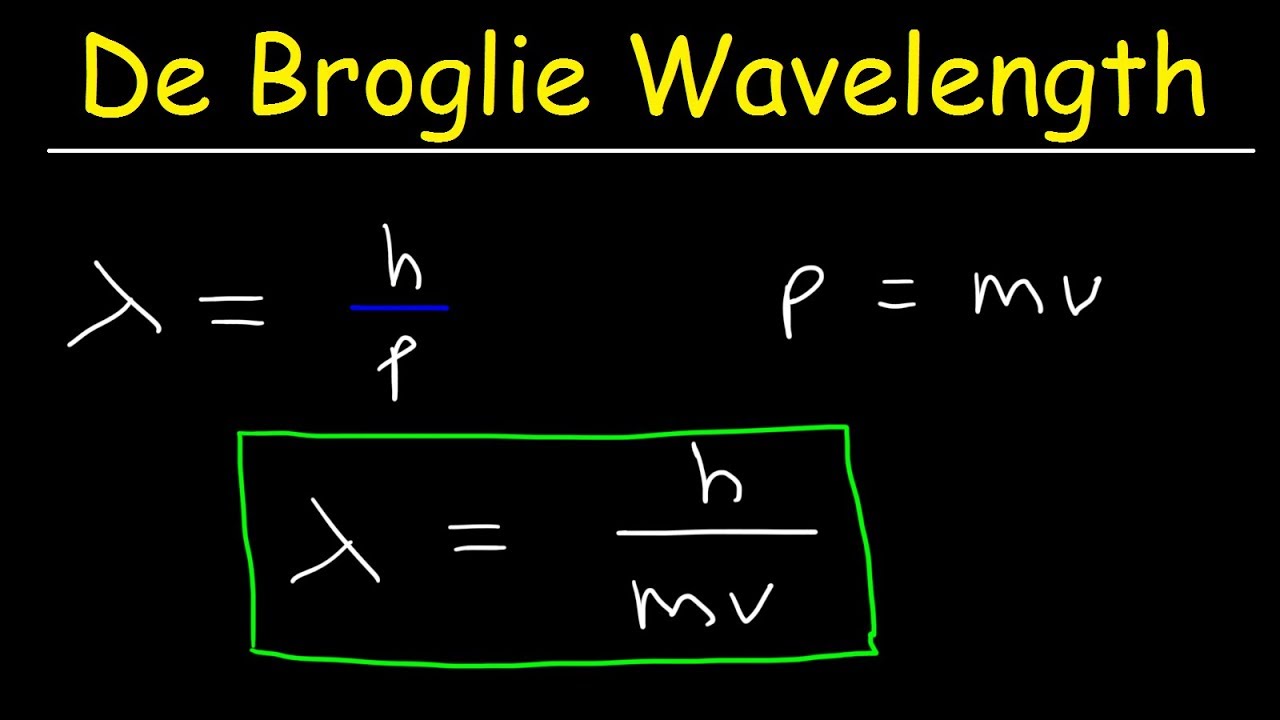
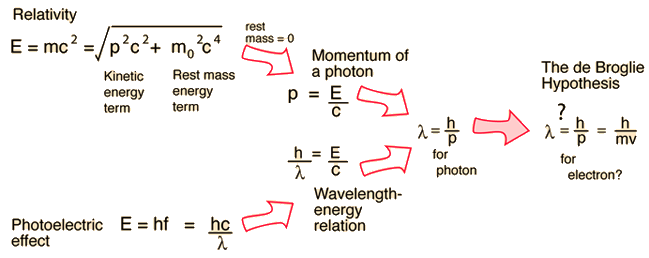
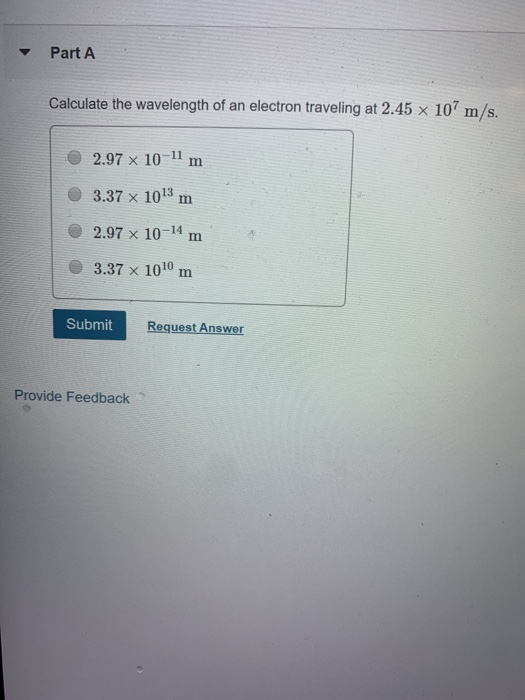
de Broglie wavelength of an electron after being accelerated by a potential difference of V volt - YouTube
Calculate wavelength of eletron if it is accelerated from rest through a potential difference of 1 KV
Calculate the wavelength of an electron moving with a velocity of 2.05 x 10^7 ms^-1. - Sarthaks eConnect | Largest Online Education Community

Calculate the de-Broglie wavelength of an electron (mass = 9.1 X ${{10}^{-31}}$ kg) moving at 1% speed of light (h = 6.63 X ${{10}^{-34}}$ kg ${{m}^{2}}$ ${{s}^{-1}}$ - CBSE Class 11 Chemistry - Learn CBSE Forum

Calculate the wavelength of an electron moving with a velocity fo ` 2. 05 xx 10^7 ms^(-1)`.... - YouTube
Welcome to Chem Zipper.com......: Calculate the speed and de Broglie wavelength of an electron that has been accelerated by a potential difference of 500 V.