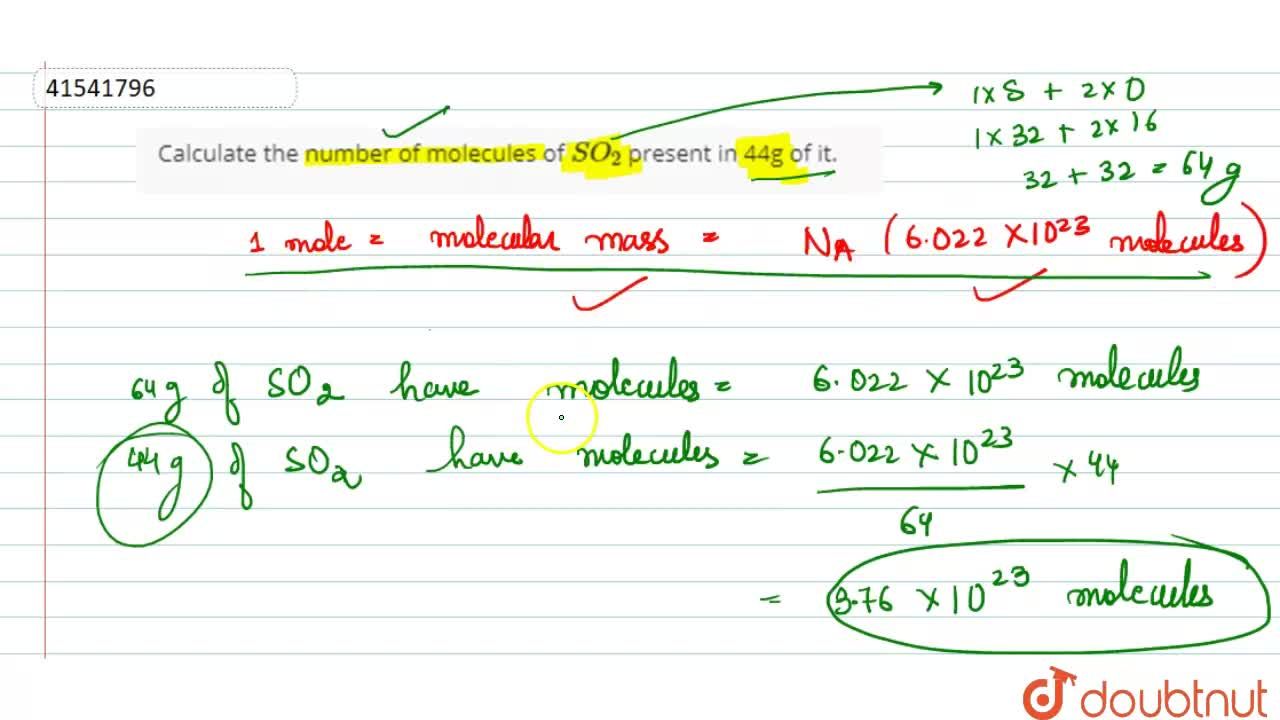
Calculate number of molecules in 3.6 g of water. | 10 | MOLE CONCEPT AND STOICHIOMETRY | CHEMIST... - YouTube

Calculate the number of moles and the number of molecules present in 1.4 g of ethylene gas. What is the volume occupied by the same amount of ethylene?A. 1.25 litresB. 1.12 litresC.
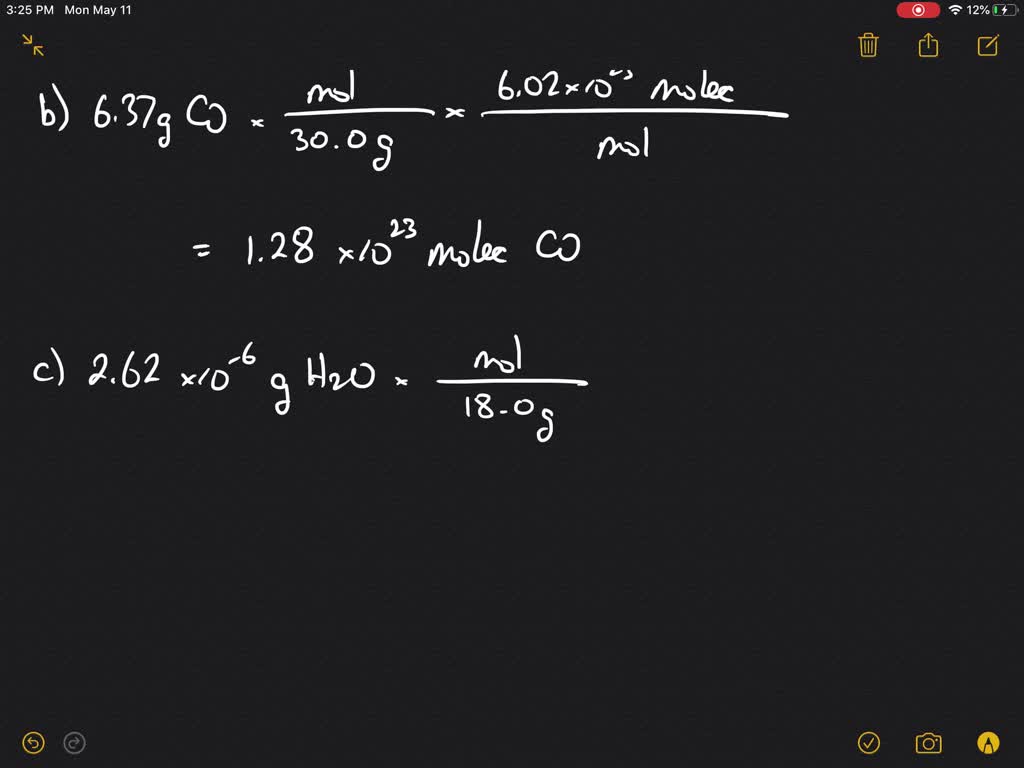
SOLVED:Calculate the number of molecules present in each of the following samples. a. 6.37 mol of carbon monoxide b. 6.37 g of carbon monoxide c. 2.62 ×10^-6 of water d. 2.62 ×10^-6
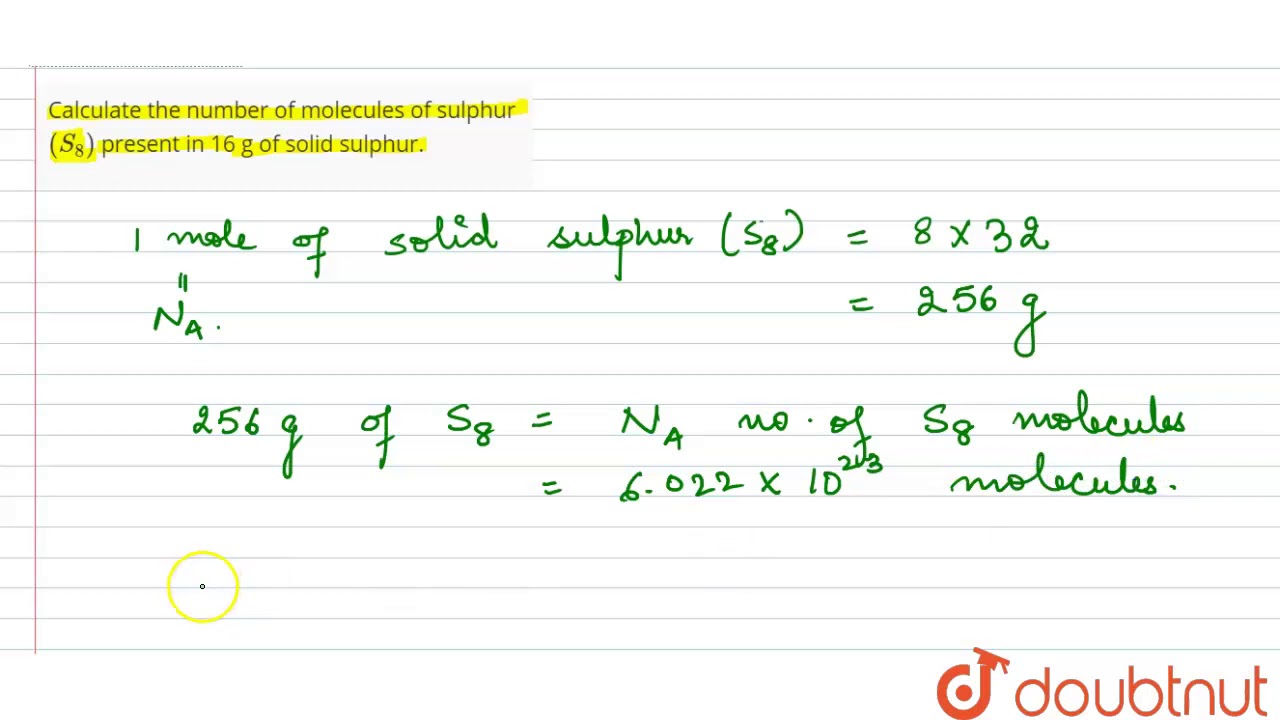
Calculate the number of molecules of sulphur `(S_(8))` present in 16 g of solid sulphur.... - YouTube

Calculate the number of moles, number of molecules and volume(V) at STP occupied 0.032 mg of CH4 (C = 12, H = 1)
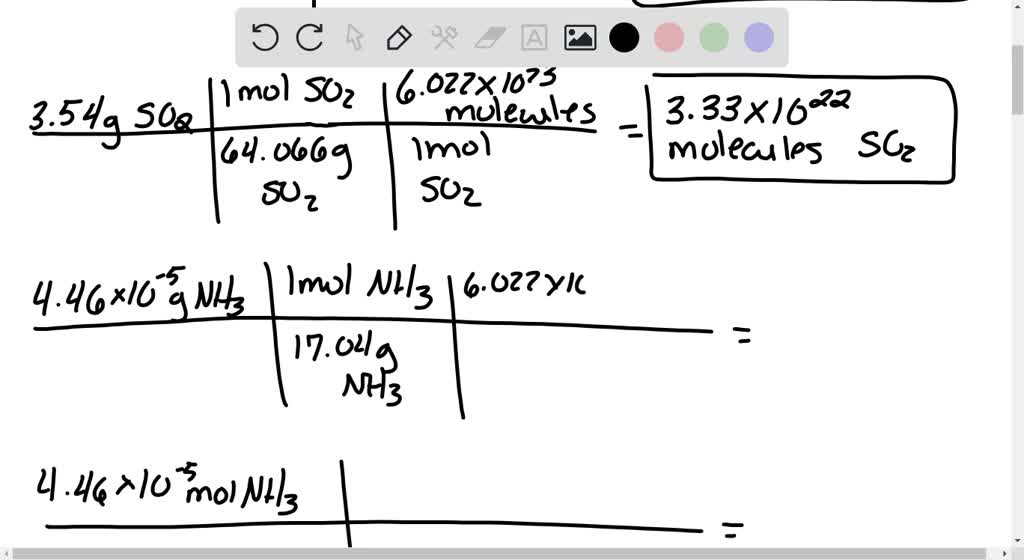
SOLVED: Calculate the number of molecules present in each of the following samples. a. 3.54 moles of sulfur dioxide, SO2 b. 3.54 g of sulfur dioxide, SO2 c. 4.46 × 10^-5 g

Calculate the number of molecules in each of the following : 1 g N2, 1 g CO2. ( Given that Na is 6.02 xx 10^23, molecular mass of N2 is 28 and CO2 is 44).
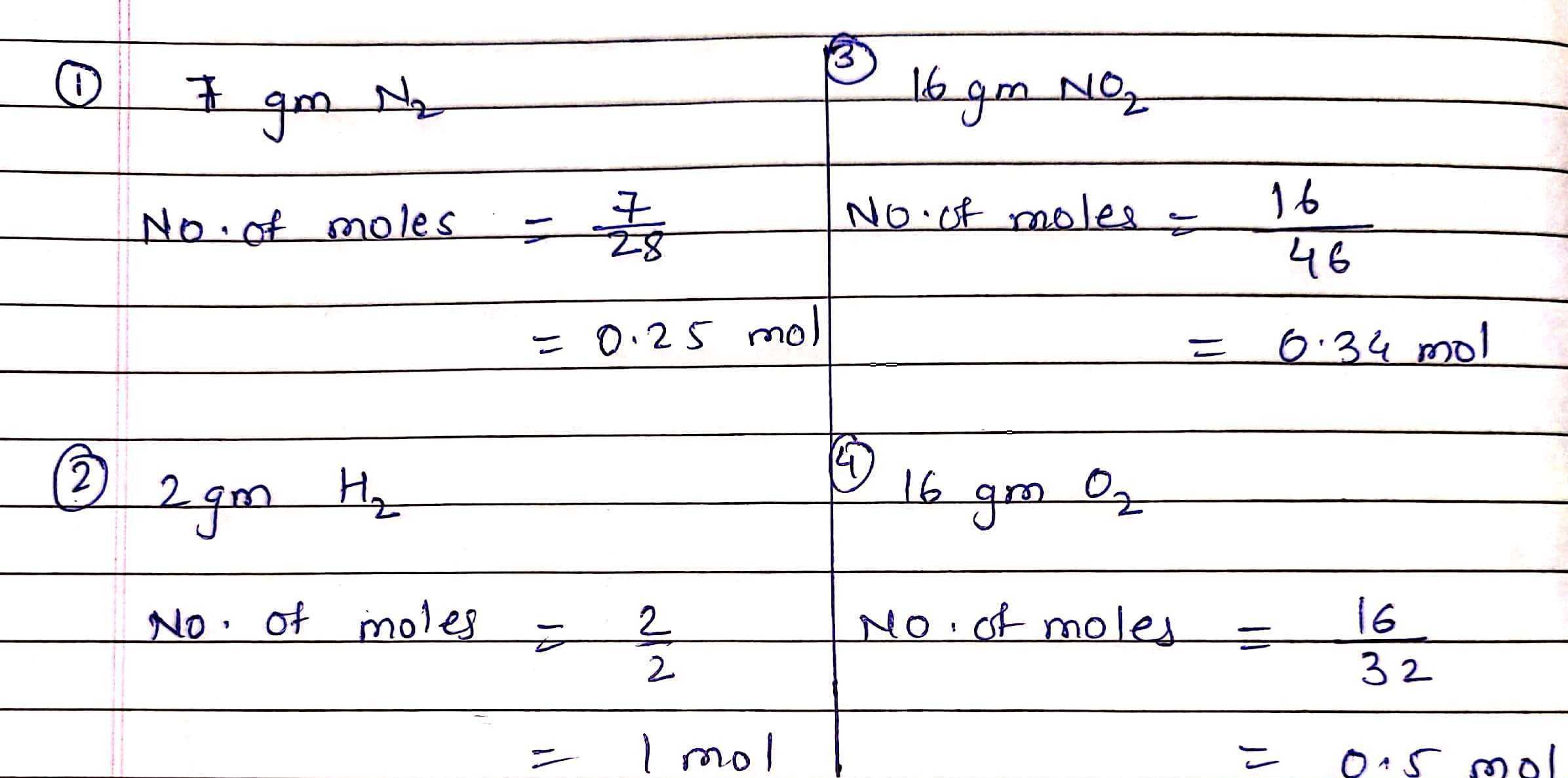
which has maximum number of molecules 1 7 gmn2 2 2 gmh2 3 16 gmno2 4 16 gmo2 8rxwz5pp -Chemistry - TopperLearning.com
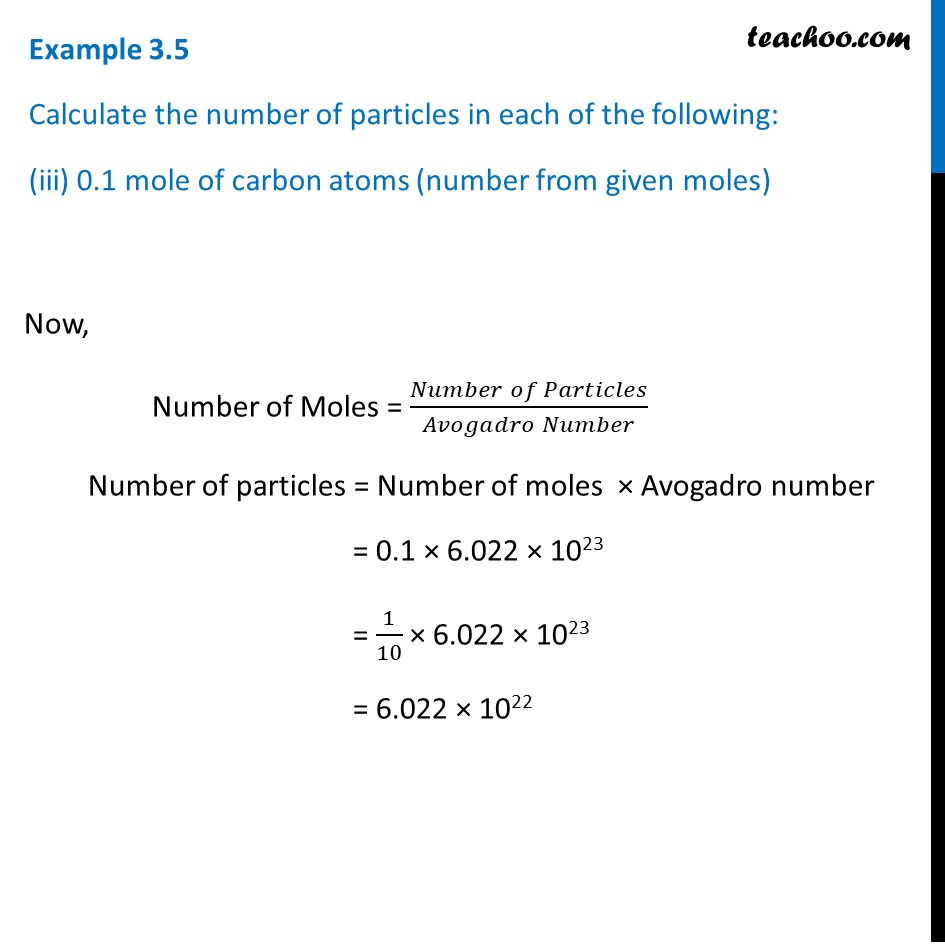
Calculate the number of particles in each of the following: (a) 48 g of Mg (b) 8 g of O2 (c) 0.1 mole of carbon (Atomic mass Mg = 24 u, O =