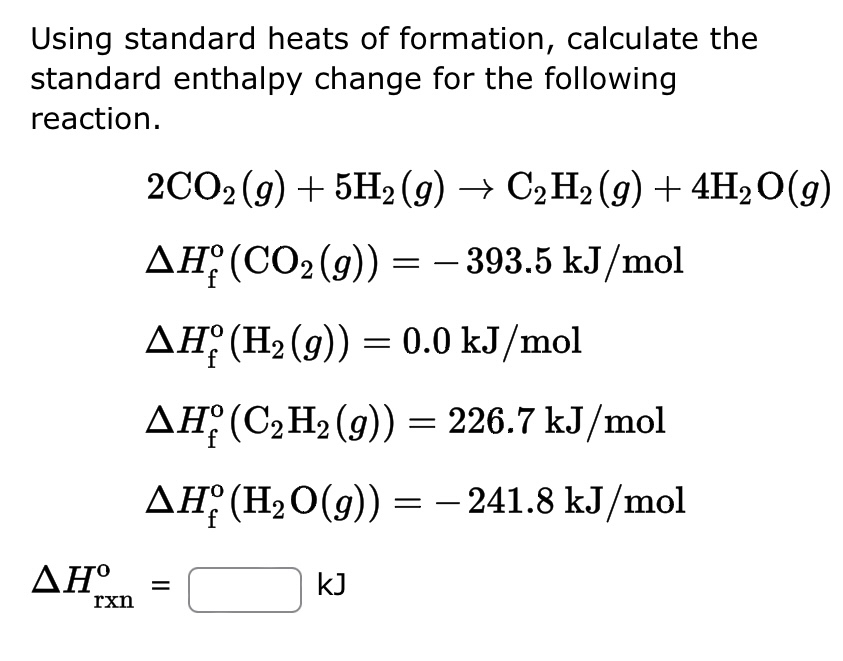
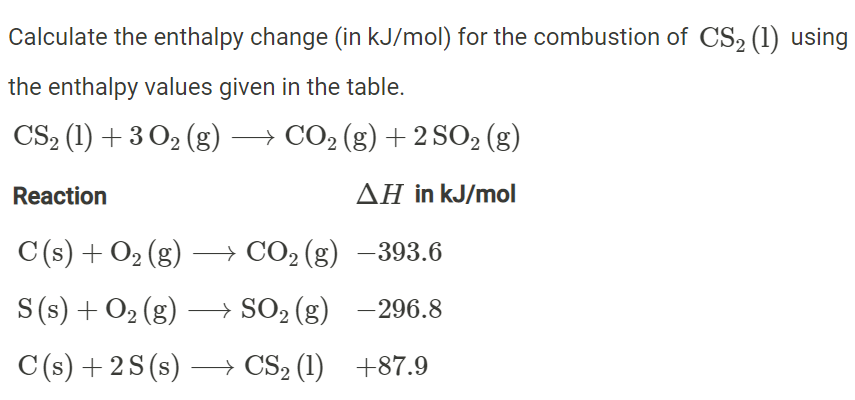Question Video: Calculating the Enthalpy Change for the Reaction between Phenol and Diatomic Hydrogen Using Standard Enthalpies of Combustion | Nagwa

Calculate the enthalpy for the reaction, H2O (g) ⟶ 2H (g) + O (g) and hence, calculate the bond enthalpy of O - H bond in H2O from the following data: ΔvapH (

Calculate the enthalpy change for the process CCl4(g)→ C(g) + 4Cl(g) and calculate bond enthalpy of C - Cl in CCl4(g) Δ vapH^ (CCl4) = 30.5 kJ mol ^-1 . Δ fH^ (
![Chemistry] Does anyone know how to calculate the enthalpy change for this reaction: : r/HomeworkHelp Chemistry] Does anyone know how to calculate the enthalpy change for this reaction: : r/HomeworkHelp](https://preview.redd.it/chemistry-does-anyone-know-how-to-calculate-the-enthalpy-v0-k0t68mmrx7n81.jpg?width=640&crop=smart&auto=webp&s=76fb8b2caf8274c7660cc18d136d2a3c28bb1764)
Chemistry] Does anyone know how to calculate the enthalpy change for this reaction: : r/HomeworkHelp