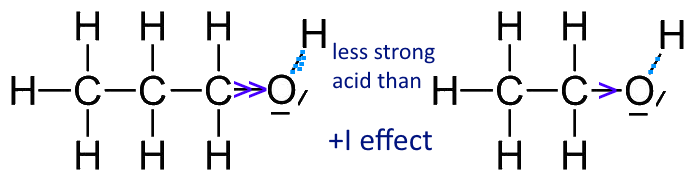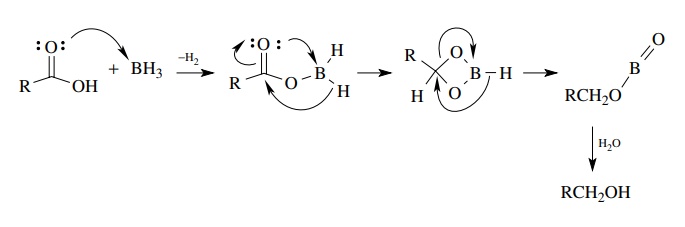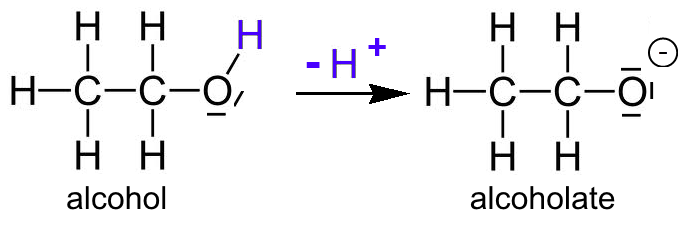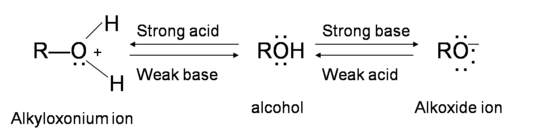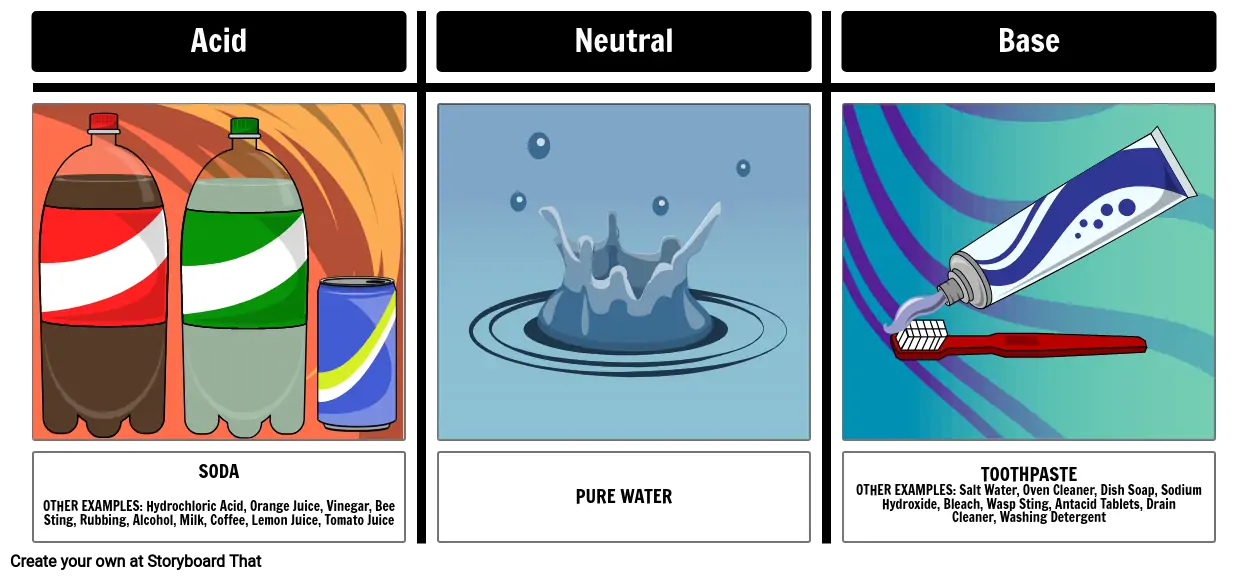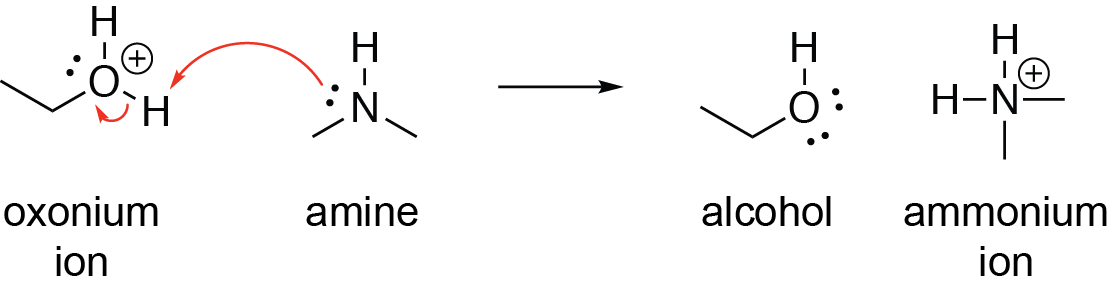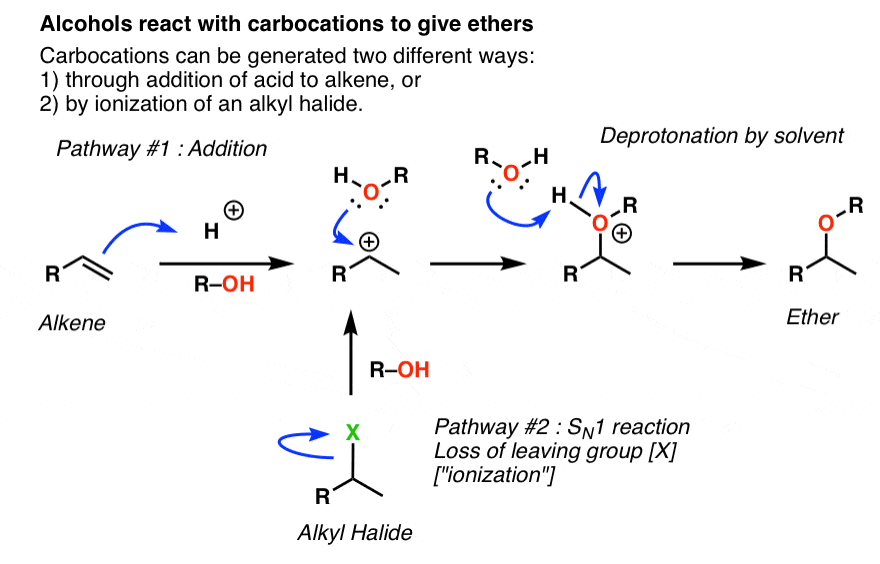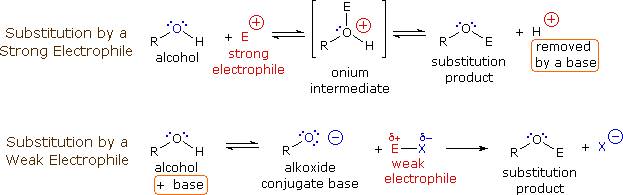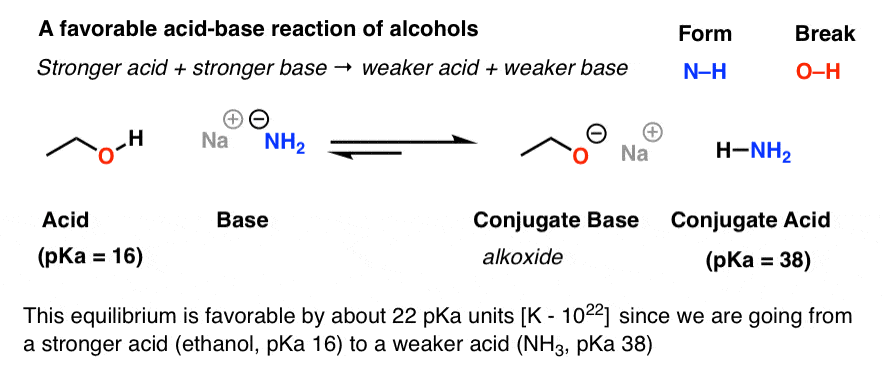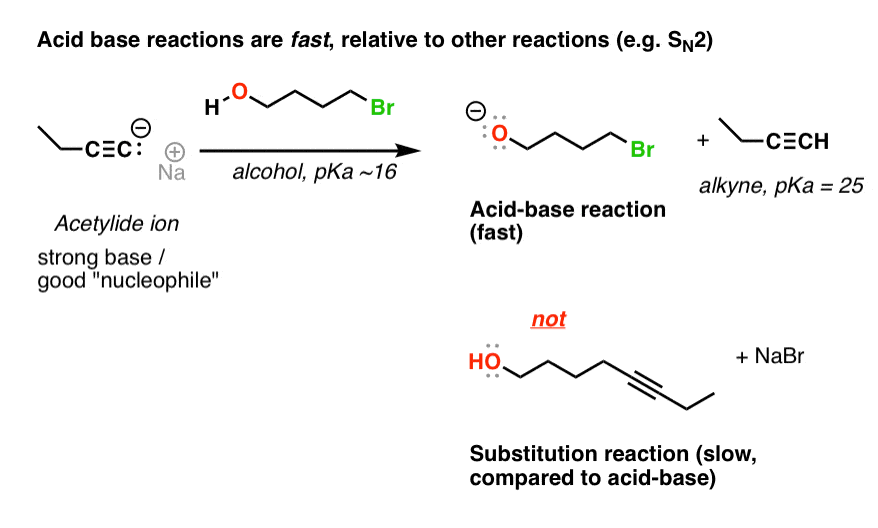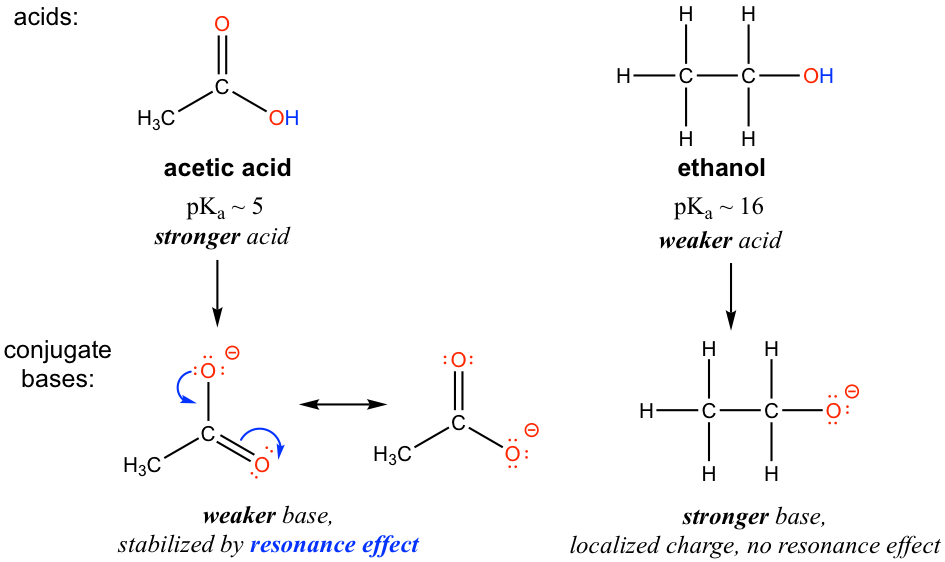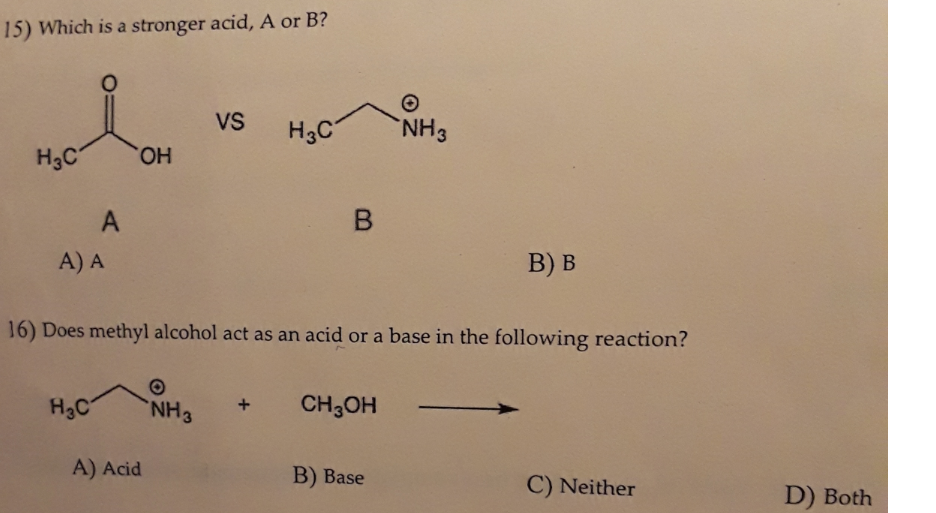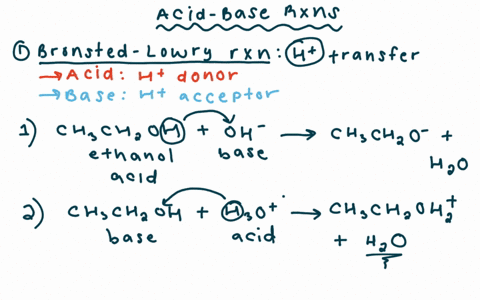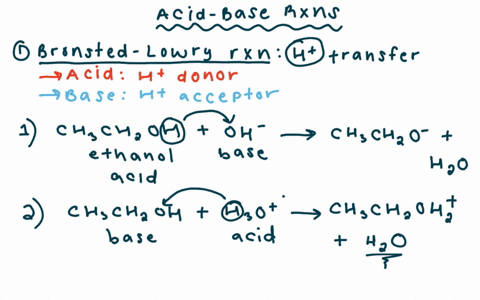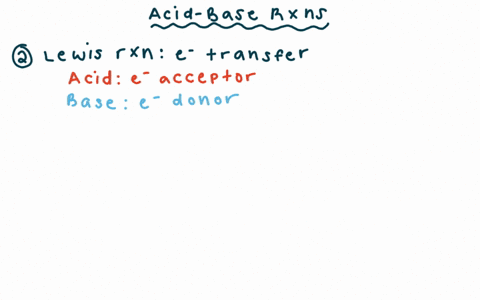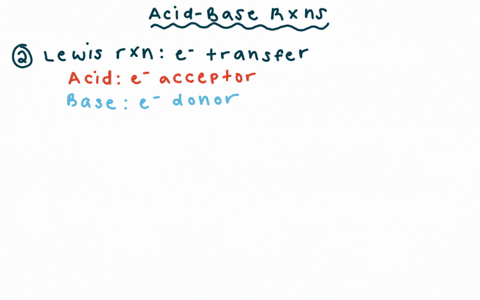
SOLVED:Ethanol (ethyl alcohol), CH3 CH2 OH, can act as a Brønsted-Lowry acid. Write the chemical equation for the reaction of ethanol as an acid with hydroxide ion, OH^-. Ethanol can also react
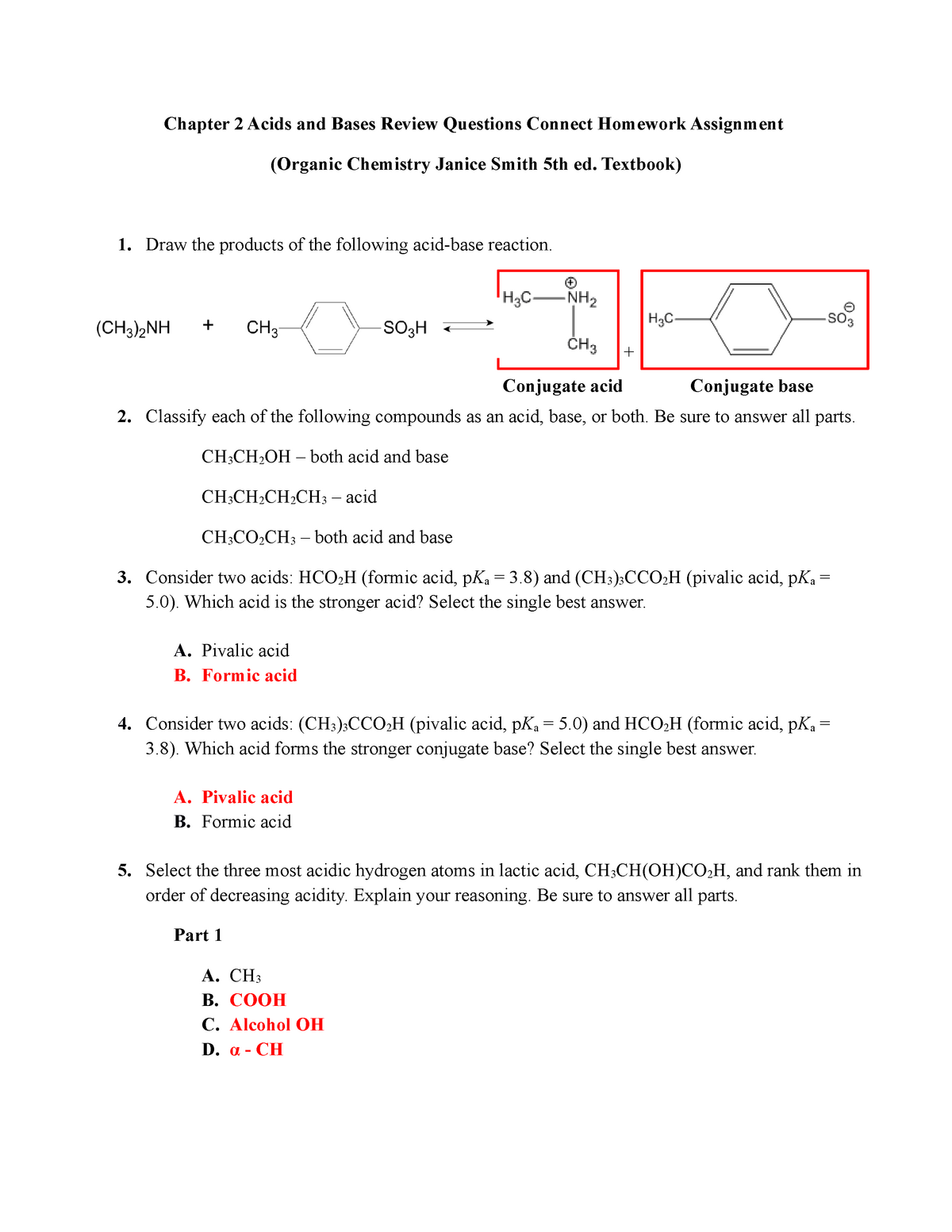
Chapter 2 Acids and Bases - Review Questions Connect Homework Assignment (Organic Chemistry Janice - Studocu